3D QSAR modeling of 4-nerolidylcatechol derivatives and virtual screening for identification of potent plasmodium inhibitor
DOI:
https://doi.org/10.3329/bjp.v9i3.18897Keywords:
3D QSAR modelling, 4-Nerolidylcatechol, Plasmodium inhibitor, Virtual screeningAbstract
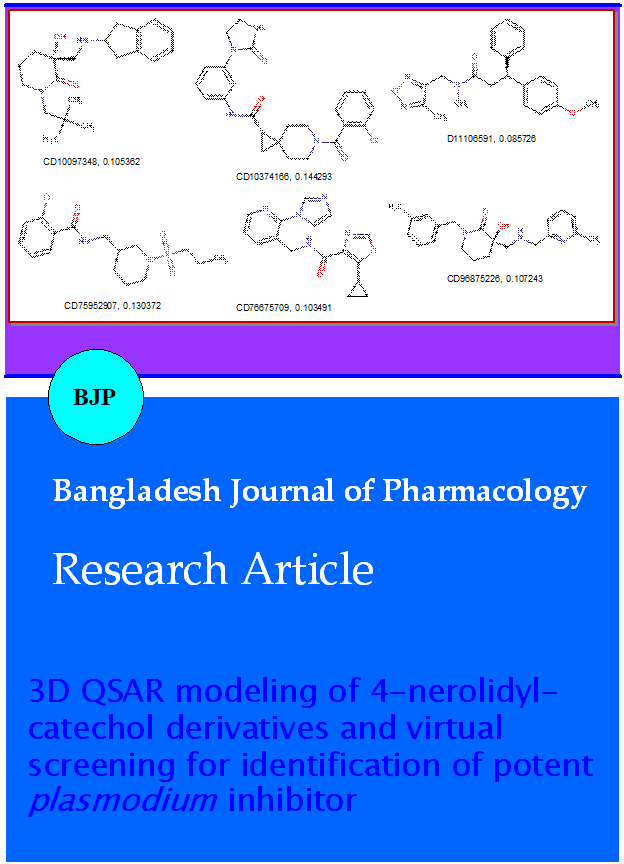
The present study was aim to develop a three dimensional quantitative structure-activity relationships (3D QSAR) model based on the structure of 4-nerolidylcatechol (IC50=0.67 µM), a novel plant derived Plasmodium inhibitor and its derivatives for identification of efficient antimalarial lead. A statisti-cally validated Partial Least-Squares (PLS) based Molecular Field Analysis (MFA) model was built up using the training set of eight 4-nerolidylcatechol derivatives and their diverse conformers. A statistically reliable model with good predictive power (cross-validated correlation coefficient q2=0.769) was obtained. Hence, the generated model was used to screen a library of 30,000 compounds of chembridge database (http://www.chembridge.com). Results of drug likeness prediction and ADMET study has suggested six compounds as potential antimalarial/plasmodial lead.
Downloads
482
431 Read
12
References
Arooj M, Thangapandian S, John S, Hwang S, Park JK, Lee KW. 3D QSAR pharmacophore modeling, in silico screening, and density functional theory (DFT) approaches for identification of human chymase inhibitors, Int J Molec Sci. 2011: 12, 9236-64.
Bagatela BS, Lopes AP, Fonseca FL, Andreo MA, Nanayakkara DN, Bastos JK, Perazzo FF. Evaluation of antimicrobial and antimalarial activities of crude extract, fractions and 4-nerolidylcathecol from the aerial parts of Piper umbellata L. (Piperaceae). Nat Prod Res. 2013; 27: 2202-09.
Bharati K, Ganguly NK. Tackling the malaria problem in the South-East Asia Region: Need for a change in policy? Indian J Med Res. 2013; 137: 36-47.
Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas C, Pierce SK. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Ann Rev Immunol. 2014; 32: 157-87.
Dearden JC. In silico prediction of drug toxicity. J Computer-aided Molec Design 2003; 17: 119-27.
Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol Today 2000; 16: 196-201.
Gogoi RR, Gogoi D, Bezbaruah RL. Virtual Screening of compounds from Tabernaemontana divaricata for potential anti-bacterial activity. Bioinformation 2014; 10: 152-56.
Mitra I, Saha A, Roy K. Pharmacophore mapping of arylamino-substituted benzo[b]thiophenes as free radical scavengers. J Molec Modeling 2010; 16: 1585-96.
Mohapatra PK, Prakash A, Bhattacharyya DR, Mahanta J. Malaria situation in north-eastern region of India, ICMR Bull. 1998; 28: 22-30.
NVBDCP. National vector borne diseases control programme.
NVBDCP, Govt. of India, 2010. Available from: www.nvbdcp.gov.in
Pinto AC, Silva LF, Cavalcanti BC, Melo MR, Chaves FC, Lotufo LV, de Moraes MO, de Andrade-Neto VF, Tadei WP, Pessoa CO, Vieira PP, Pohlit AM. New antimalarial and cytotoxic 4-nerolidylcatechol derivatives, Eur J Med Chem. 2009; 44: 2731-35.
Rocha ESLF, Silva Pinto AC, Pohlit AM, Quignard EL, Vieira PP, Tadei WP, Chaves FC, Samonek JF, Lima CA, Costa MR, Alecrim M, Andrade-Neto VF. In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phytotherapy Res. 2011; 25: 1181-88.
Scholz S, Sela E, Blaha L, Braunbeck T, Galay-Burgos M, Garcia-Franco M, Guinea J, Kluver N, Schirmer K, Tanneberger K, Tobor-Kaplon M, Witters H, Belanger S, Benfenati E, Creton S, Cronin MT, Eggen RI, Embry M, Ekman D, Gourmelon A, Halder M, Hardy B, Hartung T, Hubesch B, Jungmann D, Lampi MA, Lee L, Leonard M, Kuster E, Lillicrap A, Luckenbach T, Murk AJ, Navas JM, Peijnenburg W, Repetto G, Salinas E, Schuurmann G, Spielmann H, Tollefsen KE, Walter-Rohde S, Whale G, Wheeler JR, Winter MJ. A European perspective on alternatives to animal testing for environmental hazard identification and risk assessment. Regulatory Toxicol Pharmacol. 2013; 67: 506-30.
Silva Lima E, Silva Pinto AC, Nogueira KL, Rocha e Silva LF, Oliveira de Almeida PD, Carvalho de Vasconcellos M, Chaves FC, Tadei WP, Pohlit AM. Stability and antioxidant activity of semi-synthetic derivatives of 4-nerolidylcatechol. Molecules 2012; 18: 178-89.
Wadood A, Riaz M, Uddin R, Ul-Haq Z. In silico identification and evaluation of leads for the simultaneous inhibition of protease and helicase activities of HCV NS3/4A protease using complex based pharmacophore mapping and virtual screening. PloS one 2014; 9: e89109.
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).
