Bangabandhu Sheikh Mujib Medical University Journal
Volume 16, Issue 4, December 2023
ORIGINAL ARTICLE
Clinical and
radio-angiographic features of paediatric moyamoya disease in Bangladesh![]()
Kanij Fatema1![]() , Md Mizanur
Rahman1
, Md Mizanur
Rahman1![]() , Mohammad
Monir Hossain2
, Mohammad
Monir Hossain2![]() , Shaheen
Akhter1
, Shaheen
Akhter1![]()
1Department of Paediatric Neurology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
2Department of Paediatric Neurology, National Institute of Neuroscience and Hospital, Dhaka, Bangladesh
Correspondence to: Dr. Kanij Fatema, Email: maiomonami@gmail.com
DOI: https://doi.org/10.3329/bsmmuj.v16i4.65774
Received: 26 Apr 2023; Revised version received: 22 Oct 2023; Accepted: 6 Dec 2023
Published online: 10 December, 2023
![]()
ABSTRACT
Background: Moyamoya disease is a cerebrovascular arteriopathy of unknown origin characterized by progressive stenosis followed by occlusion of the cerebral arteries. Studies on moyamoya disease, especially in children in Bangladesh, are rare. We aimed to determine the clinical and neuroimaging features of moyamoya disease, particularly angiographic features.
Methods: Forty children diagnosed with moyamoya disease were consecutively recruited from Bangabandhu Sheikh Mujib Medical University Hospital, Dhaka, Bangladesh. Each patient underwent a medical history and physical examination focusing on stroke, magnetic resonance imaging, and magnetic resonance angiography scans of the brain. In some instances, electroencephalogram and digital subtraction angiography were also performed.
Results: Of the 40 patients, 22 experienced their first-ever stroke (median age, 84 months), and 18 had recurrent strokes (median age, 90 months). Common symptoms included hemiparesis, headache, seizure, and speech disorder. The commonly affected vessels were the internal carotid and middle cerebral arteries. Cortical involvement was found in 82.5% of cases. Bilateral involvement was observed in 37.5% of the patients, most of whom were in the Suzuki stage III.
Conclusion: Hemiparesis, headache, seizure, and speech disorder were the common manifestations. Most patients reported late (Suzuki stages III and IV), indicating an advanced stage. Early detection is necessary, considering the severity of the disease and its inherent tendency for recurrence.
Keywords: Moyamoya disease, paediatric stroke, radio-angiographic characteristics
INTRODUCTION
Moyamoya disease is an angiopathy where there is progressive occlusion of the internal carotid artery and/or its branches, causing recurrent strokes in children. In this condition, there is compensatory development of collateral blood vessels in the brain, which is pathogonomic. These collateral blood vessels have a ‘puff of smoke’ appearance on conventional angiographic imaging, which is encapsulated in the term 'moyamoya' in Japanese.1, 2 This disorder was first described in 1957 in Japan and was called moyamoya disease by Suzuki and Takaku in 1969.3 The etiopathogenesis of MMD is not known precisely. However, there are infectious, inflammatory, and genetic basis.4, 5
Moyamoya syndrome defines characteristic arterial changes that are associated with comorbid medical conditions like neurofibromatosis, Down syndrome, and sickle cell disease.6, 7 Moyamoya disease is an important cause of paediatric stroke, both ischemic and hemorrhagic. The prevalence of moyamoya disease in Japan is 0.35 to 0.94 per 1,000,000 people.8, 9 The prevalence is almost double in females compared to males. There is a bimodal distribution of age; the first peak is between 5 to 9 years and second peak is between 45 to 49 years.
The clinical manifestation of moyamoya disease exhibits distinct variations in adults and children. Adult patients predominantly experience cerebral infarctions and intracranial hemorrhages, whereas paediatric cases are more frequently associated with ischemic events. Moreover, clinical manifestations vary according to geographical distribution.10-13 Children with moyamoya disease present with diverse manifestations, thus underscoring the imperative of early diagnosis for effective management. Identifying the risk factors and clinical features of both initial and recurrent cerebrovascular events is crucial. This study has been done to identify the clinical manifestations, neuroimaging profile, and risk factors of moyamoya disease in children.
HIGHLIGHTS
1. Moyamoya disease in Bangladeshi children is not very uncommon.
2. Almost half of the children with moyamoya disease had recurrent strokes.
3. Hemiparesis, headache, seizure, and speech disorder were the common manifestations.
4. Eight in every ten patients had cortical involvement.
5. More than half of the patients presented in Suzuki stages III and IV, indicating late diagnosis. Measures for an early diagnosis are necessary.
METHODS
This cross-sectional study was done under the Department of Paediatric Neurology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, and data were collected from March 2019 to March 2021. Children under 18 years diagnosed with moyamoya disease through magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of the brain were recruited. Patients’ age, age at onset of neurological events, clinical manifestations, familial medical history, prior treatment interventions, and neuroimaging data (MRI and MRA) were documented. MRI findings were evaluated by expert radiologists. Additionally, electroencephalogram (EEG) results were collated. The therapeutic approaches administered to the patients were recorded. The diagnostic criteria for moyamoya disease adhered to the guidelines set by the International Paediatric Stroke Study, focusing on arteriopathy criteria that involve the narrowing or blockage of the distal segments of one or both internal carotid arteries, accompanied by a network of telangiectatic collateral vessels beyond the obstructed arteries.14
The MRA findings were categorized using Suzuki staging as follows: Stage I- narrowing of the carotid fork; Stage II: initiation of the moyamoya, dilated anterior cerebral artery (ACA), middle cerebral artery (MCA) and narrowed internal carotid artery (ICA) bifurcation with moyamoya change; Stage III: intensification of the moyamoya, further increase in moyamoya change of the ICA bifurcation and narrowed ACA and middle cerebral artery (MCA) ; Stage IV: minimization of the moyamoya, moyamoya change reducing with occlusive changes in ICA and tenuous ACA and MCA; Stage V: reduction of the moyamoya, further decrease in moyamoya change with occlusion of ICA, ACA and MCA; Stage VI: disappearance of the moyamoya, ICA essentially disappeared with the supply of brain from ECA. A recurrent stroke was defined as a distinct acute onset focal neurological deficit occurring >24 hours after the initial stroke. Data regarding recurrent stroke, including clinical features, age, risk factors, and neuroimaging features, were included.
Statistical analysis
Data were analyzed using SPSS version 23. The median (interquartile range) for continuous variables and number (percent) for categorical variables were presented. To compare the first and recurrent stroke groups chi-square test (for categorical variables) and Mann-Whitney U test (for continuous variables) were used. P<0.05 was considered as statistically significant.
RESULTS
Out of 40 children with MMD, 22 had the first stroke, and 18 had recurrent stroke. The median age of the children in the first stroke group was 84 months, while in the recurrent stroke group was 90 months. More than half (54.5%) of the first stroke group and two-thirds (66.7%) of the recurrent stroke group were boys. The median onset of the disease in the first and recurrent stroke groups was 27 and 54 months, respectively. No significant statistical difference was found among these two groups (TABLE 1).
The most predominant clinical features observed were hemiparesis, seizure, headache, and speech disorder. Other features were cranial nerve palsy, cognitive impairment, developmental delay and regression, and loss of consciousness. The comparison between first and recurrent stroke groups showed no significant difference in clinical features, except for developmental regression and loss of consciousness (TABLE 2).
Brain MRI scans were generally abnormal across the cases. In the group with recurrent strokes, all patients showed abnormal MRI results, whereas in the first stroke group, two individuals had normal brain MRIs. Eight in every ten patients had cortical involvement, while others had putamen, globus pallidus involvement. Cortical atrophy and fluid void were present in one-fourth of the patients. The internal carotid artery was the commonest artery which was involved. EEG results indicated that 18 patients exhibited focal discharges, while 8 had generalized discharges (TABLE 3).
At diagnosis, 57.5% of the patients were in Suzuki stage III, and 27.5% were in stage II, indicating the disorder's identification in an advanced stage, which may predict adverse outcomes. Most of the patients had Suzuki stage III involvement in MRA, while in the first episode of stroke, 25% had stage III, 22.50% had stage II involvement, while in recurrent strokes, 32.50% patients had stage III and 7.50% had stage IV involvement (FIGURE 1). MRI of brain fluid attenuated inversion recovery (FLAIR) image showing periventricular hyperintensity in one patient with MMD (FIGURE 2A) and MRI of brain T2 image showing multiple flow voids in periventricular areas suggestive of development of collateral blood vessels (FIGURE 2B). MRA of the brain shows non-visualization of internal carotid artery branches on both sides (FIGURE 3A). MRA of the brain shows multiple collaterals of internal carotid artery branches, giving the characteristic ‘puff of smoke’ appearance (FIGURE 3B).
DISCUSSION
Moyamoya disease is a chronic, occlusive disorder of cerebral vessels causing stroke in children and adults. It is an important cause of recurrent stroke in the paediatric population.15-17 The study's key finding was loss of consciousness, and developmental regression was significantly high in recurrent moyamoya disease. None of the patients in the recurrent group had a normal brain MRI. This warrants the necessity of neuroimaging for an early diagnosis. Most patients reported to the hospital in Suzuki stages III and IV.
In the current study, 45% had a recurrence in contrast to the findings of Zhao M et al. (7.4%), and Chiu D et al. (5-18% ).18, 19 The clinical features in children may differ from adults. For instance, ischemia is often observed in children, while hemorrhage is common in adults. Infarction constitutes 70-80% of moyamoya disease cases in children in previous studies.20, 21 The features in our children like infarction, headache, hemiparesis, seizure, and difficulty in speech were observed by Kim et al.22 and Lee et al.2 The predominant type of seizure in this study was focal seizure. There are studies that reported both focal and generalized seizures, probably because of hypoperfusion.23
Cognitive impairment is an important issue to address in children with moyamoya disease. It is important to know that the recurrence causes more cognitive decline compared to first-ever strokes. Hsu et al. discovered that cognitive impairment was related to temporal lobe lesions in children.24 Kurokawa et al. in their study found that four years after moyamoya disease onset, many children revive with normal intelligence levels. But it declines again in the course of time. Normal intelligence levels may be dropped in one-third of the patients after 10-15 years.25, 26 Therefore, a comprehensive assessment of cognitive function and early measures are needed to restore cognitive functions among the victims of moyamoya disease.
Developmental status is affected in children with moyamoya disease. Williams et al. in their study, showed that children with moyamoya disease scored significantly lower than the test standardization samples on all indices of intelligence and ratings of executive functioning. Moreover, children with bilateral disease and stroke scored significantly lower than those with unilateral disease in respect of intellectual function and verbal comprehension.27 This should be taken into consideration during the management planning.
There are certain characteristic signs of moyamoya disease in MRI of the brain. First, there is the Ivy sign, which refers to the appearance of the brain on postcontrast T1-weighted or FLAIR images where prominent leptomeningeal collaterals with slow blood flow and post-contrast enhancement give an Ivy-type appearance.28 The abnormal moyamoya vessels: lenticulostriate, thalamoperforating, leptomeningeal, and dural arteries, appear as multiple tortuous flow voids on T1 and T2 weighted sequences. There are multiple foci of microbleeds and also prominent deep medullary veins "brush sign" on susceptibility sequences.29 In our study cases, 38 patients had abnormal MRI of the brain. The most affected area was the cortex. Other areas involved were the thalamus, putamen, and globus pallidus. One-fourth had a fluid void in our series.
MRA is a relatively time-consuming, safer, and noninvasive modality of investigation with no requirement for contrast medium and radiation exposure. However, it may miss very small stenotic lesions.30 In a study by Yamada et al., MRA diagnosed stenosis in more than 80% of the cerebral vessels. Both MRI and MRA are very sensitive (up to 100%) and specific (up to 93%).31, 32 Bilateral involvement was another feature in four in ten patients, but Yamada et al. found it in 64% of the patients.32
The Suzuki grading system is well accepted for categorizing moyamoya disease. It indicates the intrinsic compensatory reorganization of stenosis of the ICA, subsequent development of moyamoya vessels, compensatory development of trans-dural/trans-cranial anastomosis from the external carotid artery system, and finally, disappearance of moyamoya vessels at the late stage.33, 34 In this study more than half of the cases presented in Suzuki stage III which reflected the wide gap of time to diagnose the cases. It also indicates that some of the features of MMD were ignored. Therefore, measures are necessary to promote early detection and treatment.
A seizure in moyamoya disease is an important tool for diagnosing facal or generalized epileptic discharge. In a similar study, Frechette et al. found that 90% of EEGs were abnormal in patients with moyamoya disease. The majority of them had focal epileptic discharges.35
Conclusion
Although rare, moyamoya disease is an important cause of childhood stroke. Cerebral infarction, headache, hemiparesis, dysarthria, and seizure are common manifestations. Recurrence of stroke is very common in paediatric moyamoya disease. MRI and MRA are important diagnostic tools. The cortex of the brain is the most common site of stroke occurrence. Patients in our series reported to the hospital very late, having Suzuki stages lll and lV. Therefore, an early detection is necessary, considering the severity of the disease and its inherent tendency for recurrence.
Acknowledgments
We would like to thank the department of Radiology and Imaging for their support in reporting the neuroimaging, study participants and our residents for their active participation.
Author Contributions
Conception and design: KF, MMR, SA. Acquisition, analysis and interpretation of data: KF, MMH. Manuscript drafting and revising critically: KF, MMR, MMH, SA. Approval of final version of the manuscript: KF, MMR, MMH, SA. Guarantor accuracy and integrity of work: KF, MMR, MMH, SA.
Funding
No funds were received for this study.
Conflicts of Interest
The authors have no conflict of interest to declare.
Ethical Approval
The parent of all subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board (IRB) of Bangabandhu Sheikh Mujib Medical University (Memo no: BSMMU/2019/11856).
ORCID iDs
Kanij Fatema https://orcid.org/0000-0002-2465-053X
Md Mizanur Rahman https://orcid.org/0000-0002-9225-7617
Mohammad Monir Hossain https://orcid.org/0000-0001-6790-5048
Shaheen Akhter https://orcid.org/0000-0003-1222-0193
REFERENCES
1. Guey S, Tournier-Lasserve E, Hervé D, Kossorotoff M. Moyamoya disease and syndromes: from genetics to clinical management. Appl Clin Genet. 2015 Feb 16;8:49-68. DOI: https://doi.org/10.2147/TACG.S42772.
2. Lee S, Rivkin MJ, Kirton A, deVeber G, Elbers J; International Pediatric Stroke Study. Moyamoya Disease in Children: Results From the International Pediatric Stroke Study. J Child Neurol. 2017 Oct;32(11):924-929. DOI: https://doi.org/10.1177/0883073817718730.
3. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969 Mar;20(3):288-299. DOI: https://doi.org/10.1001/archneur.1969.00480090076012.
4. Ikeda H, Sasaki T, Yoshimoto T, Fukui M, Arinami T. Mapping of a familial moyamoya disease gene to chromosome 3p24.2-p26. Am J Hum Genet. 1999 Feb;64(2):533-537. DOI: https://doi.org/10.1086/302243.
5. Yamada H, Deguchi K, Tanigawara T, Takenaka K, Nishimura Y, Shinoda J, Hattori T, Andoh T, Sakai N. The relationship between moyamoya disease and bacterial infection. Clin Neurol Neurosurg. 1997 Oct;99 Suppl 2:S221-S224. DOI: https://doi.org/10.1016/s0303-8467(97)00048-6.
6. Ibrahimi DM, Tamargo RJ, Ahn ES. Moyamoya disease in children. Childs Nerv Syst. 2010 Oct;26(10):1297-1308. DOI: https://doi.org/10.1007/s00381-010-1209-8.
7. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009 Mar 19;360(12):1226-1237. DOI: https://doi.org/10.1056/NEJMra0804622.
8. Wakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, Kojima M, Lin Y, Ohno Y. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997 Oct;99 Suppl 2:S1-S5. DOI: https://doi.org/10.1016/s0303-8467(97)00031-0.
9. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008 Aug;79(8):900-904. DOI: https://doi.org/10.1136/jnnp.2007.130666.
10. Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000 Sep;20 Suppl:S61-S64. DOI: https://doi.org/10.1046/j.1440-1789.2000.00300.x.
11. Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004 Feb;100(2 Suppl Pediatrics):142-149. DOI: https://doi.org/10.3171/ped.2004.100.2.0142.
12. Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of moyamoya disease in the United States. Stroke. 1998 Jul;29(7):1347-1351. DOI: https://doi.org/10.1161/01.str.29.7.1347.
13. Hallemeier CL, Rich KM, Grubb RL Jr, Chicoine MR, Moran CJ, Cross DT 3rd, Zipfel GJ, Dacey RG Jr, Derdeyn CP. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke. 2006 Jun;37(6):1490-1496. DOI: https://doi.org/10.1161/01.STR.0000221787.70503.ca.
14. Houkin K, Nakayama N, Kuroda S, Nonaka T, Shonai T, Yoshimoto T. Novel magnetic resonance angiography stage grading for moyamoya disease. Cerebrovasc Dis. 2005;20(5):347-354. DOI: https://doi.org/10.1097/01.mop.0000144441.29899.20.
15. Kim JS. Moyamoya Disease: Epidemiology, Clinical Features, and Diagnosis. J Stroke. 2016 Jan;18(1):2-11. DOI: https://doi.org/10.5853/jos.2015.01627.
16. Fujimura M, Bang OY, Kim JS. Moyamoya Disease. Front Neurol Neurosci. 2016;40:204-220. DOI: https://doi.org/10.1159/000448314.
17. Huang S, Guo ZN, Shi M, Yang Y, Rao M. Etiology and pathogenesis of Moyamoya Disease: An update on disease prevalence. Int J Stroke. 2017 Apr;12(3):246-253. DOI: https://doi.org/10.1177/1747493017694393.
18. Zhao M, Deng X, Gao F, Zhang D, Wang S, Zhang Y, Wang R, Zhao J. Ischemic Stroke in Young Adults with Moyamoya Disease: Prognostic Factors for Stroke Recurrence and Functional Outcome after Revascularization. World Neurosurg. 2017 Jul;103:161-167. DOI: https://doi.org/10.1016/j.wneu.2017.03.146.
19. Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of moyamoya disease in the United States. Stroke. 1998 Jul;29(7):1347-1351. DOI: https://doi.org/10.1161/01.str.29.7.1347.
20. Takanashi J. Moyamoya disease in children. Brain Dev. 2011 Mar;33(3):229-234. DOI: https://doi.org/10.1016/j.braindev.2010.09.003.
21. Yonekawa Y. [Operative neurosurgery: personal view and historical backgrounds (9) Moyamoya angiopathy (MMA): past history and status presens]. No Shinkei Geka. 2012 Jan;40(1):67-87. Japanese. PMID: 22223526.
22. Kim DS, Ko TS, Ra YS, Choi CG. Postoperative electroencephalogram for follow up of pediatric Moyamoya disease. J Korean Med Sci. 2006 Jun;21(3):495-499. DOI: https://doi.org/10.3346/jkms.2006.21.3.495.
23. Zheng W, Wanibuchi M, Onda T, Liu H, Koyanagi I, Fujimori K, Houkin K. A case of moyamoya disease presenting with chorea. Childs Nerv Syst. 2006; 22:274–278. DOI: https://doi.org/10.1007/s00381-004-1104-2.
24. Hsu YH, Kuo MF, Hua MS, Yang CC. Selective neuropsychological impairments and related clinical factors in children with moyamoya disease of the transient ischemic attack type. Childs Nerv Syst. 2014 Mar;30(3):441-447. DOI: https://doi.org/10.1007/s00381-013-2271-9.
25. Kurokawa T, Tomita S, Ueda K, Narazaki O, Hanai T, Hasuo K, Matsushima T, Kitamura K. Prognosis of occlusive disease of the circle of Willis (moyamoya disease) in children. Pediatr Neurol. 1985 Sep-Oct;1(5):274-277. DOI: https://doi.org/10.1016/0887-8994(85)90027-x.
26. Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004 Feb;100(2 Suppl Pediatrics):142-149. DOI: https://doi.org/10.3171/ped.2004.100.2.0142.
27. Williams TS, Westmacott R, Dlamini N, Granite L, Dirks P, Askalan R, Macgregor D, Moharir M, Deveber G. Intellectual ability and executive function in pediatric moyamoya vasculopathy. Dev Med Child Neurol. 2012 Jan;54(1):30-DOI: https://doi.org/10.1111/j.1469-8749.2011.04144.x.
28. Savolainen M, Pekkola J, Mustanoja S, Tyni T, Hernesniemi J, Kivipelto L, Tatlisumak T. Moyamoya angiopathy: radiological follow-up findings in Finnish patients. J Neurol. 2020 Aug;267(8):2301-2306. DOI: https://doi.org/10.1007/s00415-020-09837-w.
29. Horie N, Morikawa M, Nozaki A, Hayashi K, Suyama K, Nagata I. "Brush Sign" on susceptibility-weighted MR imaging indicates the severity of moyamoya disease. AJNR Am J Neuroradiol. 2011 Oct;32(9):1697-16702. DOI: https://doi.org/10.3174/ajnr.A2568.
30. Saeki N, Silva MN, Kubota M, Takanashi J, Sugita K, Nakazaki S, Yamaura A. Comparative performance of magnetic resonance angiography and conventional angiography in moyamoya disease. J Clin Neurosci. 2000 Mar;7(2):112-115. DOI: https://doi.org/10.1054/jocn.1999.0160.
31. Fujiwara H, Momoshima S, Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005 Aug;55(2):224-230. DOI: https://doi.org/10.1016/j.ejrad.2004.11.009.
32. Yamada I, Suzuki S, Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging versus conventional angiography. Radiology. 1995 Jul;196(1):211-218. DOI: https://doi.org/10.1148/radiology.196.1.7784569.
33. Suzuki J, Kodama N. Moyamoya disease--a review. Stroke. 1983 Jan-Feb;14(1):104-109. DOI: https://doi.org/10.1161/01.str.14.1.104.
34. Fujimura M, Tominaga T. Diagnosis of moyamoya disease: international standard and regional differences. Neurol Med Chir (Tokyo). 2015;55(3):189-193. DOI: https://doi.org/10.2176/nmc.ra.2014-0307.
35. Frechette ES, Bell-Stephens TE, Steinberg GK, Fisher RS. Electroencephalographic features of moyamoya in adults. Clin Neurophysiol. 2015 Mar;126(3):481-485. DOI: https://doi.org/10.1016/j.clinph.2014.06.033.
|
||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
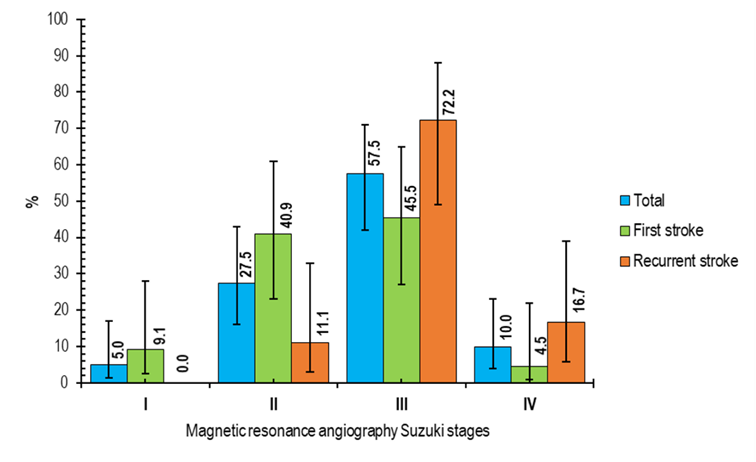
FIGURE 1 Magnetic resonance angiography Suzuki stages of children with moyamoya disease (n=40)
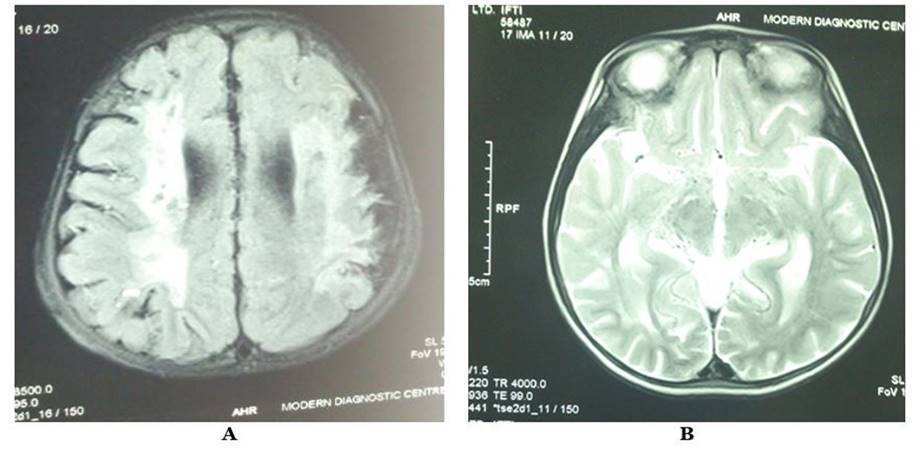
FIGURE 2 Magnetic resonance imaging (A and B) of children with moyamoya disease
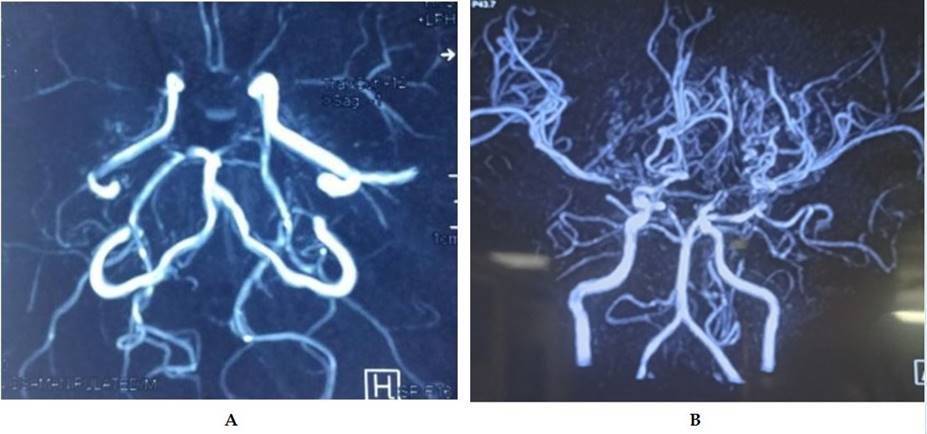
FIGURE 3 Magnetic resonance angiography (A and B) of children with moyamoya disease.
(c) 2023 The Authors. Published by BSMMU Journal