Bangabandhu Sheikh Mujib Medical University Journal
Volume 16, Issue 4, December 2023
BRIEF ARTICLE
Expression
of Ki-67 and E-cadherin in patients with non-small cell lung cancer attending a
tertiary care hospital![]()
Farzana Afroze1![]() , Latifa Nishat1
, Latifa Nishat1![]() , Farida Arjuman2
, Farida Arjuman2![]() , Zinnat Ara Yesmin1
, Zinnat Ara Yesmin1![]() , Lutfun Nahar1
, Lutfun Nahar1![]() , Rokhsana Tanjin1
, Rokhsana Tanjin1![]()
1Department of Anatomy, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
2Department of Histopathology, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh
DOI: https://doi.org/10.3329/bsmmuj.v16i4.68719
Received: 25 July 2023; Revised version received: 21 Oct 2023; Accepted: 22 Oct 2023
Published online: 26 November, 2023
![]()
ABSTRACT
Background: E-cadherin and Ki-67 expressions may provide real-time insights into the tumor's status and can be utilized as targeted therapeutics for lung cancer. We aimed to explore the expression of Ki-67 and E-cadherin in non-small cell lung cancer (NSCLC) patients and to identify their association with clinicopathological features.
Methods: In this cross-sectional study, forty formalin-fixed paraffin-embedded (FFPE) NSCLC tissue blocks were identified from January to October 2022, based on hospital records from the Department of Pathology of National Institute of Diseases of the Chest and Hospital, Dhaka Medical College and Hospital. Samples were reevaluated for tissue quality, diagnosis, and exclusion-inclusion criteria. Finally, twenty-five samples were analyzed, and relevant clinicopathological data were collected from patients or authorized representatives. Ki-67 and E-cadherin expression were analyzed by immunohistochemistry and their relationships with each other.
Results: Ki-67 expression was positive in 40% of the NSCLC tissue, and E-cadherin was negative in 40% of the NSCLC tissue. No statistically significant relation was found between the expressions of Ki-67 and E-cadherin. No statistically significant association was found in Ki-67 and E-cadherin expressions with clinicopathological characteristics except E-cadherin with comorbidity.
Conclusion: Positive expression of Ki-67 and negative expression of E-cadherin was found in two-fifths of NSCLC tissues but not significant. Simultaneous estimated of Ki-67 and E-cadherin may contribute to the treatment planning and predict prognosis of the NSCLC patients.
Keywords: Ki-67, E-cadherin, NSCLC, immunohistochemistry, epithelial-mesenchymal transition
INTRODUCTION
Lung cancer stands as a predominant cause of cancer-related death globally. According to the cancer registry report (2018-2020), it occupied the first position (17.4%) among the cancers occurring in both sexes in Bangladesh.1 It is thought that the failure of lung cancer treatment is primarily attributed to metastasis and recurrence, resulting in a persistently dim prognosis for this condition.2 Around 80% of total lung cancers are non-small cell lung cancer (NSCLC).3 The molecular mechanism of lung cancer is very complex. Immunohistochemistry (IHC) plays an important role in characterizing cancer cells by identifying various biomarkers. One of the molecular processes involved in lung cancer is known as epithelial-mesenchymal transition (EMT), a complex reprogramming process of epithelial cells, which plays an essential role in tumor invasion and metastasis. Loss of E-cadherin (a cell-to-cell adhesion molecule) expression is one of the hallmarks of EMT.4 E-cadherin is a transmembrane glycoprotein expressed on the cell surface and maintains cell-cell junctions, thereby inhibiting aberrant cell proliferation and migration.5
For assessment of tumor proliferation, there is a frequent reliance on the analysis of proliferation-associated antigens, particularly Ki-67, a nuclear protein.3 It is expressed throughout all cell cycle phases in actively proliferating cells, but it is not expressed in quiescent (G0) cells. As a result, Ki-67 can serve as an ideal target antigen for evaluating proliferation in NSCLC.6 Therefore, concurrent assessment of E-cadherin and Ki-67 can provide real-time insights into the tumor's status and can be utilized as targeted therapeutics for lung cancer, as proposed by He et al.7 We aimed to explore the expression of Ki-67 and E-cadherin in NSCLC patients and to identify their association with clinicopathological features.
HIGHLIGHTS
1. Positive expression of Ki-67 was found in two-fifths of NSCLC tissues.
2. Negative expression of E-cadherin was found in two-fifths of NSCLC tissues.
3. A combination of E-cadherin and Ki-67 assessment can be useful for therapeutics in lung cancer.
METHODS
Study design and samples
This cross-sectional study was done under the Department of Anatomy of the Bangabandhu Sheikh Mujib Medical University. The data were collected from January 2022 to October 2022. Initially, a total of 40 formalin-fixed paraffin-embedded (FFPE) NSCLC tissue blocks were identified based on hospital records from the Department of Pathology at the National Institute of Diseases of the Chest and Hospital and Dhaka Medical College and Hospital. Simultaneously, respective patients were communicated for further information. A total of 15 tissue samples were excluded due to insufficient tissue, prior chemotherapy or radiotherapy, and absence of patient records. Twenty-five samples were analyzed, and relevant clinicopathological data, including age, sex, and associated histopathological reports, were collected from the patients or their authorized representatives after obtaining written informed consent. Laboratory analysis was performed at the Histopathology Department of the National Institute of Cancer Research and Hospital.
Identification of tumor specimens
FFPE tissue blocks were cut into 4micro meter thick sections and incubated in a hot air oven at 60-650C for 30 minutes. Then, clearing was done in xylene (three changes) and rehydrated gradually in decreasing concentrations (100%, 90%, 80%, 70%) of isopropyl alcohol. The slides were treated with Dako target retrieval solution in a conventional water bath and rinsed with Tris buffer solution (TBS; pH 7.4). Then, the slides were incubated in 100-150 micro L PRB (peroxidase blocking reagent) to block the endogenous peroxidase activity. Then the slides were incubated with primary antibodies, Ki-67 (FLEX monoclonal Mo A Hu Ki-67 Antigen, MIB-1, Dako, Denmark) and E-cadherin (FLEX monoclonal Mo A Hu E-cadherin Antigen, NCH-38, Dako, Denmark) for 30 minutes. Then, the slides were washed in TBS and incubated in horseradish peroxidase blocking reagent for 30 minutes. After that, staining and counter-staining were done with diaminobenzidine and hematoxylin, respectively. Positive controls (vermiform appendix for Ki-67 and normal breast tissue for E-cadherin) were stained as described for tumor specimens.8
Validation of immunohistochemical staining
One of us (FA) scored the slides and validated them with a Histopathologist (FA). Ki-67 positive cells were identified based on brown-stained nuclei. The counting was done by identifying hot spots or by global method.9 The area with the highest number of Ki-67 positive nuclei was considered a hot spot. Positive status depends on the number of brown stained nuclei, not staining intensities.10 Four hot spots of Ki-67 positive cells were identified (if clearly seen), and counted at least 100 nuclei in each field. If the hot spots were unavailable in the slide, the global method was followed (one field with the highest Ki-67 index containing 500 nuclei). The total number of Ki-67 positive nuclei was counted and divided by the total number of counted cancer cells under high power magnification (x400). Finally, the Ki-67 index was expressed as a percentage. Ki-67 expression was divided into two groups: ≤ 5% considered Ki-67 negative or low, and > 5% as Ki-67 positive or high. For E-cadherin expression, the degree of positive staining of tumor cells was scored as follows: 1 (≤10% stained cells), 2 (11-30% stained cells), 3 (31-60% stained cells), 4 (> 60% stained cells). The staining intensity of tumors was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). Then, the stained cells' scores (0-4) and the color intensity (0-3) of each specimen were multiplied, and a final score of 0 to 12 was achieved. The final score of 0 to 3 was considered negative, and the 4 to 12 was considered an E-cadherin positive sample.7
Statistical analysis
Statistical analysis was done using SPSS, version 25. Data were described in terms of both number and percent. Fisher's exact test was done to assess the relationship between the expressions of E-cadherin and Ki-67. All statistical tests were two-tailed, and P<0.05 was considered statistically significant.
RESULTS
The patients of the selected samples had a mean (standard deviation) age of 56.8 (10.1) years, with 84% being male. Two-fifths had at least one comorbid medical condition, and two in every ten had a family history of lung cancer. More than half of the samples (56%) exhibited squamous cell carcinoma, and nearly half (48%) of the tumors were well-differentiated. Lymph node metastasis was present in one out of every ten samples.
Expressions of Ki-67 and E-cadherin are given in FIGURE 1 and FIGURE 2, respectively. Of the 25 NSCLC tissue samples, 15 exhibited positive expression for E-cadherin, while 15 were negative for Ki-67 expression. Of the 15 NSCLC tissues with positive E-cadherin expression, Ki-67 was positive in 6 cases. Conversely, among the 10 NSCLC tissues negative for E-cadherin, Ki-67 was positive in 4 cases. However, no statistically significant association (P=0.99) was identified between Ki-67 and E-cadherin expression (TABLE 1). No statistically significant association was found in the expressions of both Ki-67 and E-cadherin with age, sex, family history of lung cancer, histological type, tumor differentiation, and lymph node metastasis except E-cadherin expression and co-morbidity of the patients (P=0.01).
DISCUSSION
Ki-67 is considered a molecular marker that reflects the tumor's aggressiveness and can anticipate the prognosis of NSCLC patients. We observed positive expression of Ki-67 was found in two-thirds of NSCLC tissues, which was almost similar to the study by Lin et al. (38%).11 Positive expression of Ki-67 was found in most of the NSCLC samples in studies done by Folescu et al.12 in Europe Ahn et al.13 in Korea and Hommura, et al. in Japan.14
E-cadherin maintains the core of the epithelial adherens junction with neighboring cells. Fei et al.15 recommended that reduced expression of this molecule is responsible for developing malignant phenotype in NSCLC. E-cadherin expression was negative in two-thirds of patients NSCLC tissue. Our finding is almost in line with Kim et al.,16 who found a negative expression of E-cadherin in one-fifth of the samples. Wrona et al.17 and Chao et al.18 found that over half of the NSCLC tissue samples showed downregulation of E-cadherin. Another study in Korea showed downregulation of E-cadherin in 78.4% of NSCLCcases.19 Poorly differentiated tumors show more positive and less negative expression of Ki-67 and E-cadherin, respectively. Most of the tumors were well differentiated in this present study. This may be the probable explanation for more negative expression of Ki-67 and more positive expression of E-cadherin in this study.
He et al.7 and Grigoras et al.20 revealed an inverse correlation between the expression of E-cadherin and Ki-67 in NSCLC. These findings demonstrated that detecting lung cancer aggressiveness may benefit from potential correlations between E-cadherin and Ki-67 and assessing their level simultaneously. The present study's findings are inconsistent with these studies, possibly due to the small sample size and low percentage of poorly differentiated tumors analyzed in this study.
This study found no statistically significant association between Ki-67 and E-cadherin expression with clinicopathological characteristics. A statistically significant association was found between E-cadherin expression and co-morbidity of the patients; the NSCLC patients with comorbidity (Chronic obstructive pulmonary disease/asthma) exhibited positive expression of E-cadherin in this study. On the other hand, Ki-67 expression did not show any statistically significant association with co-morbidity. No statistically significant association was found between Ki-67 and E-cadherin expression with the age, sex, histological type, tumor differentiation, lymph node metastasis, and family history of lung cancer. Our finding is consistent with the findings of Yang et al.,19, 21 who did not find any significant correlation between E-cadherin expression with age, sex, and tumor histologic type. However, a study in Korea found a considerable correlation between the down-regulation of E-cadherin and socio-demographic characteristics like male gender and histological type19. He et al.7 and Yang et al.21 found a significant association of negative expression of E-cadherin with lymph node metastasis and tumor differentiation.
Ki-67 expression may be considered a valuable marker for aggressive NSCLC and lung cancer prognosis, as suggested by a few studies.6, 12, 13, 22 These studies found a significant correlation between high Ki-67 expression and clinicopathological features such as age, gender, tumor differentiation, histological subtype, and lymph node metastasis. However, we need an extended study to explore the issue.
We acknowledge some limitations of the study. We used surgically resected or core biopsy NSCLC tissue but did not get enough tissue for some of the FFPE core biopsy. The sample employed in this study is not representative of the wider population. As seen by other studies, the sample size is also relatively small to detect real differences.
Conclusion
We observed that positive expression of Ki-67 and negative expression of E-cadherin was found in two-fifths of NSCLC tissues but not significant as most of the tumors were well differentiated without distant metastasis and with minimum lymph node involvement. Simultaneous estimated of Ki-67 and E-cadherin may contribute to the treatment planning and predict prognosis of the NSCLC patients.
Acknowledgments
We want to thank the authority of the Department of Pathology of Department of Pathology at the National Institute of Diseases of the Chest and Hospital (NIDCH) and Dhaka Medical College and Hospital for their cooperation during sample collection. We also acknowledge Dr. Md. Delwar Hossain, Associate Professor, Thoracic Surgery, NIDCH, and thank the study population for their active participation.
Author Contributions
Conception and design: FA, LN. Acquisition, analysis and interpretation of data: FA, FA, RT, LN. Manuscript drafting and revising it critically: FA, LN, ZAY. Approval of the final version of the manuscript: FA, LN, FA, ZAY, LN, RT. Guarantor accuracy and integrity of the work: FA, LN.
Funding
This research received institutional resources provided by the Department of Anatomy, and the research grant awarded by Bangabandhu Sheikh Mujib Medical University.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
All subjects gave informed consent for inclusion before participating in the study. The study was conducted as per the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board (IRB) of Bangabandhu Sheikh Mujib Medical University (No. BSMMU/2022/6959; Date: 18-07-2022) and the IRB of National Institute of Cancer Research and Hospital (No. NICRH/Permission/2022/232/1(6).
ORCID iDs
Farzana Afroze https://orcid.org/0009-0009-5936-5614
Latifa Nishat https://orcid.org/0000-0003-1393-6927
Farida Arjuman https://orcid.org/0009-0001-7010-7423
Zinnat Ara Yesmin https://orcid.org/0000-0002-2163-6126
Lutfun Nahar https://orcid.org/0009-0008-8723-7081
Rokhsana Tanjin https://orcid.org/0009-0004-4440-491X
References
1. Hospital Cancer Registry Report 2018-2020, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh; Accessed 26 January, 2023 [Internet]; Available from: https://nicrh.gov.bd/images/reports/41272-hbcr-2018-2020-report_compressed.pdf.
2. Wu Y, Liu HB, Ding M, Liu JN, Zhan P, Fu XS, Lu G. The impact of E-cadherin expression on non-small cell lung cancer survival: a meta-analysis. Mol Biol Rep. 2012 Oct;39(10):9621-9628. DOI: https://doi.org/10.1007/s11033-012-1827-1.
3. Warth A, Cortis J, Soltermann A, Meister M, Budczies J, Stenzinger A, Goeppert B, Thomas M, Herth FJ, Schirmacher P, Schnabel PA, Hoffmann H, Dienemann H, Muley T, Weichert W. Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer. 2014 Sep 9;111(6):1222-1229. DOI: https://doi.org/10.1038/bjc.2014.402.
4. Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004 Aug 6;118(3):277-279. DOI: https://doi.org/10.1016/j.cell.2004.07.011.
5. Heimann R, Lan F, McBride R, Hellman S. Separating favorable from unfavorable prognostic markers in breast cancer: the role of E-cadherin. Cancer Res. 2000 Jan 15;60(2):298-304. PMID: 10667580.
6. Wen S, Zhou W, Li CM, Hu J, Hu XM, Chen P, Shao GL, Guo WH. Ki-67 as a prognostic marker in early-stage non-small cell lung cancer in Asian patients: a meta-analysis of published studies involving 32 studies. BMC Cancer. 2015 Jul 15;15:520. DOI: https://doi.org/10.1186/s12885-015-1524-2.
7. He LY, Zhang H, Wang ZK, Zhang HZ. Diagnostic and prognostic significance of E-cadherin and Ki-67 expression in non-small cell lung cancer patients. Eur Rev Med Pharmacol Sci. 2016 Sep;20(18):3812-3817. PMID: 27735037.
8. Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society's standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014 Oct;62(10):693-697. DOI: https://doi.org/10.1369/0022155414545224.
9. Kucukosmanoglu I, Karanis MIE, Unlu Y, Erol M. Correlation of [18F]FDG PET activity with expressions of Ki-67 in non-small-cell lung cancer. Nucl Med Rev Cent East Eur. 2022;25(2):73-77. DOI: https://doi.org/10.5603/NMR.a2022.0017.
10. Paul TK, Banu PA, Alam MSS, Sharif R, Rukhsana N, Monower MM. The overview of cancer patients attending a specialized hospital: A cross sectional study. Bangladesh Med Res Counc Bull. 2015 Aug;41(2):95-100. DOI: https://doi.org/10.3329/bmrcb.v41i2.29990.
11. Lin L, Cheng J, Tang D, Zhang Y, Zhang F, Xu J, Jiang H, Wu H. The associations among quantitative spectral CT parameters, Ki-67 expression levels, and EGFR mutation status in NSCLC. Sci Rep. 2020 Feb 26;10(1):3436. DOI: https://doi.org/10.1038/s41598-020-60445-0.
12. Folescu R, Levai CM, Grigoraş ML, Arghirescu TS, Talpoş IC, Gindac CM, Zamfir CL, Poroch V, Anghel MD. Expression and significance of Ki-67 in lung cancer. Rom J Morphol Embryol. 2018;59(1):227-233. PMID: 29940632.
13. Ahn HK, Jung M, Ha SY, Lee JI, Park I, Kim YS, Hong J, Sym SJ, Park J, Shin DB, Lee JH, Cho EK. Clinical significance of Ki-67 and p53 expression in curatively resected non-small cell lung cancer. Tumour Biol. 2014 Jun;35(6):5735-5740. DOI: https://doi.org/10.1007/s13277-014-1760-0.
14. Hommura F, Dosaka-Akita H, Mishina T, Nishi M, Kojima T, Hiroumi H, Ogura S, Shimizu M, Katoh H, Kawakami Y. Prognostic significance of p27KIP1 protein and ki-67 growth fraction in non-small cell lung cancers. Clin Cancer Res. 2000 Oct;6(10):4073-4081. PMID: 11051259.
15. Fei Q, Zhang H, Chen X, Wang JC, Zhang R, Xu W, Zhang Z, Zou W, Zhang K, Qi Q, Wang M, Tao S, Luo Z. Defected expression of E-cadherin in non-small cell lung cancer. Lung Cancer. 2002 Aug;37(2):147-152. DOI: https://doi.org/10.1016/s0169-5002(02)00077-6.
16. Kim H, Yoo SB, Sun P, Jin Y, Jheon S, Lee CT, Chung JH. Alteration of the E-Cadherin/β-Catenin Complex Is an Independent Poor Prognostic Factor in Lung Adenocarcinoma. Korean J Pathol. 2013 Feb;47(1):44-51. DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.44.
17. Wrona A, Sejda A, Dziadziuszko R, Jassem J. Prognostic Significance of Wnt1, Wnt2, E-Cadherin, and β-catenin Expression in Operable Non-small Cell Lung Cancer. J Histochem Cytochem. 2021 Nov;69(11):711-722. DOI: https://doi.org/10.1369/00221554211048550.
18. Chao D, Hu G, Li Q. Clinicopathological significance and prognostic value of E-cadherin expression in non-small cell lung cancer: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021 Feb 19;100(7):e24748. DOI: https://doi.org/10.1097/MD.0000000000024748.
19. Myong NH. Reduced expression of E-cadherin in human non-small cell lung carcinoma. Cancer Res Treat. 2004 Feb;36(1):56-61. DOI: https://doi.org/10.4143/crt.2004.36.1.56.
20. Grigoras M, Cornianu M, Lazar E, Cornea R and Popescu R. E-cadherin expression in non-small cell lung carcinomas. Ann Rom Soc Cell Biol. 2012;17(1):332-341, accessed 26 January, 2023,Retrived from: https://www.researchgate.net/publication/264891411_ E-cadherin_expression_in_non-small_cell_lung_carcinomas [check the format]
21. Yang YL, Chen MW, Xian L. Prognostic and clinicopathological significance of downregulated E-cadherin expression in patients with non-small cell lung cancer (NSCLC): a meta-analysis. PLoS One. 2014 Jun 30;9(6):e99763. DOI: https://doi.org/10.1371/journal.pone.0099763.
22. Wei DM, Chen WJ, Meng RM, Zhao N, Zhang XY, Liao DY, Chen G. Augmented expression of Ki-67 is correlated with clinicopathological characteristics and prognosis for lung cancer patients: an up-dated systematic review and meta-analysis with 108 studies and 14,732 patients. Respir Res. 2018 Aug 13;19(1):150. DOI: https://doi.org/10.1186/s12931-018-0843-7.
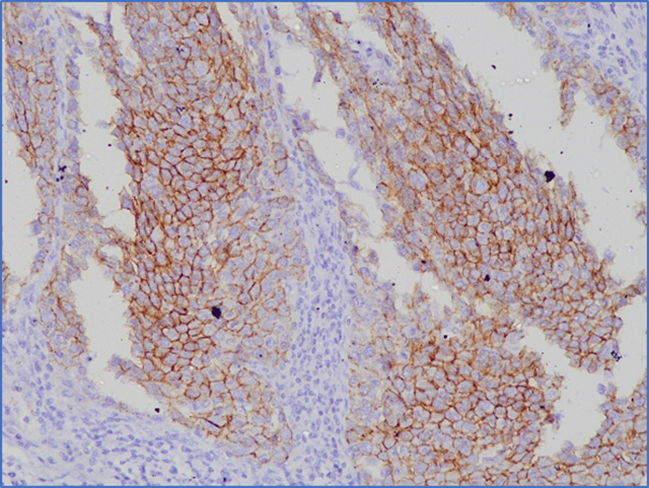
FIGURE 1 Expression of Ki-67 in non-small cell lung cancer tissue
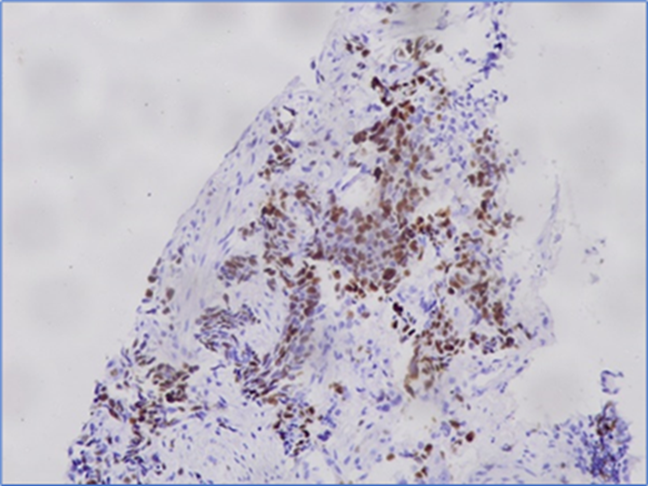
FIGURE 2 Expression of E-cadherin in non-small cell lung cancer tissue
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) 2023 The Authors. Published by BSMMU Journal