|
Bangabandhu Sheikh Mujib Medical University Journal Volume 17, Issue 1, March 2024
ORIGINAL ARTICLE Effect of
sidestream cigarette smoking on memory of male Long-Evans rats
Amina
Begum1
1Department of Physiology Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh 2Department of Physiology, Central Medical College and Hospital, Cumilla, Bangladesh 3Department of Physiology; Dhaka Medical College, Dhaka, Bangladesh
DOI: https://doi.org/10.3329/bsmmuj.v17i1.68731 Received: 15 Oct 2024; Revised version received: 14 Mar 2024; Accepted: 25 Mar 2024 Published online: 30 Mar 2024 Responsible
Editor: M Mostafa Zaman |
ABSTRACT
Background: Memory impairment is an important presentation of many diseases. Sidestream cigarette smoke (SCS), a form of passive smoke, causes neural complications such as impaired memory. The aim of this study was to assess the effect of sidestream cigarette smoke on memory of male Long-Evans rats.
Methods: This experimental study was conducted in the Physiology Department of Bangabandhu Sheikh Mujib Medical University. Twelve male Long-Evans rats, having 150 to 200 grams body weight were collected from central animal house of this University. Rats were divided into fresh air group (control) and experimental group (exposer to SCS for 30 minutes twice daily) for 30 consecutive days. For memory evaluation, Morris water maze (MWM) test was performed. Working memory was measured as escape latency in training and four trial phases. Reference memory (escape latency in acquisition phase and target crossings in probe trial. For estimation of hippocampal antioxidant enzymes, catalase and glutathione peroxidase levels were measured by ELISA. Data were expressed as mean (standard error of mean) and t test was done to compare the two groups. P<0.05 was considered as statistically significant.
Results: Two to five times higher escape latency (working memory) was observed in experimental rats compared to those of control rats (P<0.001). Moreover, significantly lower (3.8 versus 7.8) target crossings (P<0.001) were found in experimental rats compared to the control rats. In addition, hippocampal catalase (6.2 versus 17.6 U/mg protein) and glutathione peroxidase (1.9 versus 5.6 U/mg protein) levels were found significantly lower (P<0.001) in experimental rats when compared to control rats.
Conclusion: The sidestream cigarette smoke caused memory impairment and decrement of hippocampal antioxidant enzymes level in male Long-Evans rats.
INTRODUCTION
Sidestream cigarette smoke (SCS) is highly toxic because it contains numerous toxic products as a result of incomplete combustion of slowly burning cigarettes.1-4 Several neuro-complications have been reported as complications of SCS, such as ischemic stroke,5 depression,6 insufficient sleep syndrome,7 Alzheimer’s disease8 and cognitive as well as emotional impairment.9
Memory impairment is an important presentation of many diseases. Several mechanisms have been proposed as the causes of this memory impairment such as, antioxidant opposing forces.10 Superoxide dismutase catalyzes the dismutation of superoxide into hydrogen peroxide, which is then neutralized by catalase or by glutathione peroxidase.11 Exposure to cigarette smoke was previously reported to induce lung oxidative stress in mice and oxidative DNA damage to the human lymphocyte.12
Cigarettes typically have high doses of carcinogenic polycyclic aromatic hydrocarbons, volatile aldehydes, nitric oxide, carbon monoxide, and nicotine.13 High levels of carbon monoxide inhaled during sidestream cigarette smoking in human sometimes are enough to cause acute toxicity14 and induce oxidative stress in human and animals,5 thus probably influencing cognitive function.10
To examine the memory in animal model, Morris water maze (MWM) has been widely used to investigate working memory as well as reference memory.15 This task is based upon the premise that animals have evolved an optimal strategy to explore their environment and escape from the water with the minimum amount of effort.16 On the basis of this background, the present study to examine the effect of sidestream cigarette smoke on memory in male Long-Evans rats.
HIGHLIGHTS
1. Male Long-Evens rats were exposed to sidestream cigarette smoke.
2. Memory assessment was done by Morris water maze test.
3. The sidestream cigarette smoking impaired the memory of rats.
METHODS
This experimental study was conducted in the KM Fariduddin Animal Research Lab of the Department of Physiology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, from January 2023 to June 2023. The involvement of the animal subjects follow the guidelines for the Animal Experimentation Ethics Committee of International Centre for Diarrhoeal Disease Research, Bangladesh and was approved by the Institutional Review Board of BSMMU.
Long-Event rats
Twelve male rats of 8th to 10th weeks weighing 150 to 200 gram were obtained from the central animal house of BSMMU, Dhaka. All rats were kept in the KM Fariduddin Animal Research Lab of the Department of Physiology, BSMMU and was housed in specially constructed plastic cages with 3 to 4 rats per cage under a 12/12-hour light/ dark cycle.17 The room temperature was kept between 270C to 280C, corresponding to the thermoneutral zone for rodents.18 All rats had access to standard laboratory food19 cooled boiled water ad libitum during acclimatization. In order to avoid circadian influences all experiments were carried out between 08:00 and 16:00 hours.20
Experimental design
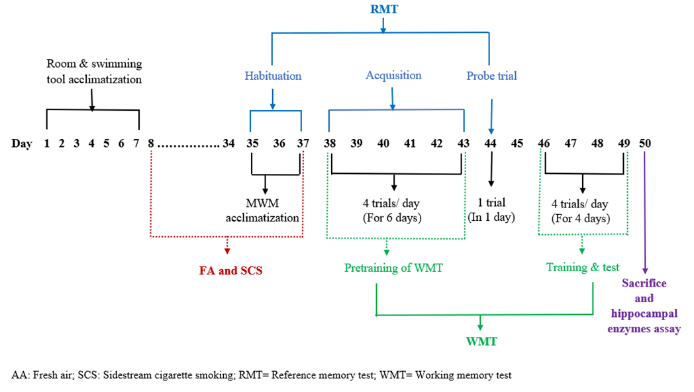
Six rats were randomly assigned to two groups: control (kept in fresh air for 30 consecutive days) and experimental (exposed to SCS for 30 minutes twice daily in morning and evening for 30 consecutive days) (figure 1).
SCS exposure
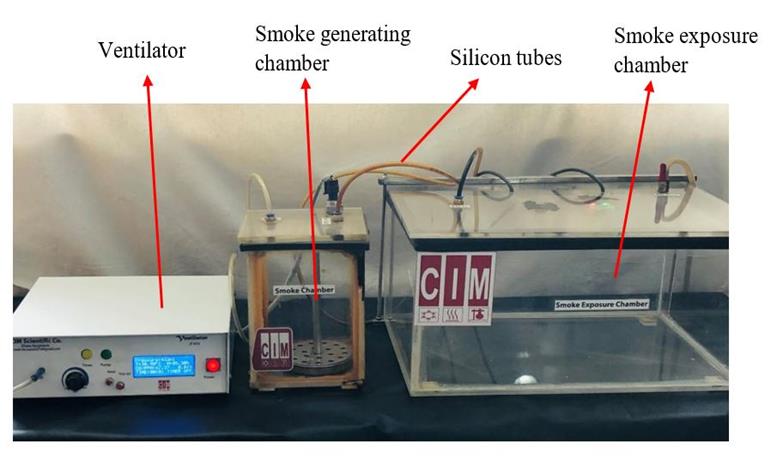
Cigarette smoke exposure (CSE) system (CIM Scientific Co, Bangladesh) consisted of a ventilator, smoke generating chamber, and smoke exposure chamber that consecutive days are serially connected via silicon tubes (figure 2). SCS was generated by burning of two cigarettes for 30 minutes twice daily at morning and evening for 30 consecutive days. Here, 150 ml of air for every 10 seconds was ventilated through the ventilator of CSE system to smoke generating chamber to create optimum positive pressure at temperature 260 C and humidity 60% and carbon monoxide concentration 150-200 ppm in smoke exposure chamber.21
Memory performance test
Rats were tested for memory using a MWM, a circular pool, 150 cm in diameter and 50 cm high.23 This pool was arbitrarily divided into four quadrants: north-west (NW), north-east (NE), south-east (SE) and south-west (SW) quadrants. A black round platform (15 cm in diameter) was placed at the center of any one quadrant. To determine the start locations, eight points north (N), south (S), east (E), west (W), north-east (NE), north-west (NW), south-east (SE) and south-west (SW) of MWM was labelled. The whole inner wall of the pool and platform was painted in black to avoid visual clue in the pool. During the test, the maze was filled with water to a depth of 30 cm with 240 to 260 C water. As a result, the platform was submerged 2 cm under the water and was the only escape place from the water for the rats. MWM test was conducted in a well illuminated room which contained numerous extra maze cues, such as a window, a door, shelves, a refrigerator, and a clock. Here the rats had to swim in water pool to search a hidden (submerged 2 cm under the water surface) platform for escape.
This experiment was divided into reference and working memory test. The reference memory test by MWM was done in three phases, composed of habituation, acquisition, and probe trial. For habituation, each rat was habituated to the water pool by swimming for 3 minutes without platform daily for three consecutive days. In acquisition phase, a rat had to swim to a prefixed platform position to escape whatever its start locations were in MWM. For this, every day every rat was brought into the laboratory for four trials (1, 2, 3, and 4) separated by 30 seconds for six consecutive days. Here, the platform was submerged at the centre of randomly prefixed north-east (NE) quadrant of the pool and was same for all trials in those days. Rats were randomly sequenced. Trial 1 was started after 30 days of sidestream cigarette smoke exposure and swimming exercise based on group assignment. Data were recorded as escape latency (time to find out the platform) in each trial. Probe trial phase was observed 24 hours after trial 4 of acquisition phase.
In this phase, the rat had to swim to a prefixed platform position from where the platform was removed. In this trial, the start location was the distant most position from the platform position. Here, the start location was SW and the platform was removed from NE quadrant. Data were recorded as number of target (platform position) crossings within 60 seconds and time spent in target in seconds within 60 seconds. The working memory test by MWM was done in two phases, composed of pre-training (six days) and training (four days). For every rat, previously completed acquisition phase of reference memory test was considered as the pre-training phase of working memory test. In training phase, a rat had to swim to a prefixed platform position to escape the location of which was changed daily. For this, everyday every rat was brought into the laboratory for four trials (1—4) separated by 30 seconds for four consecutive days. The platform was submerged 2 cm under the water surface at the center of NE quadrant of the pool for day 1 but was changed every day for four days. On the first day, the platform was placed on NE quadrant and the start locations were from SE, SW, S and W point for four trials sequentially. In each trial, the test procedure of acquisition phase of reference memory test was followed. Data were recorded as escape latency (time to find out) in all trial.
Hippocampal antioxidant enzymes assay
After sacrificing the rats, brain tissue was quickly collected according to the procedures of a previous research.24 Next, the hippocampus was dissected out from rat’s brain according to the procedures of previous research. Then, scooping of hippocampus was done and placed it in petri dish and minced into small pieces.25 To remove excess blood, all the pieces were rinsed in ice cold phosphate buffer solution (0.1 M, pH=7.40). Later, the tissue pieces were weighed and homogenized in phosphate buffer solution according to the ratio of weight (gm): volume (mL) =1:4 with a glass homogenizer on ice. Then the homogenate was centrifuged for 10 minutes at 3500 rpm to get the supernatant. The supernatant was taken in a test tube and stored at -200C 25 until hippocampal antioxidant enzymes assay. Hippocampal total protein, catalase, and glutathione peroxidase concentration in the recovered supernatant was measured using a commercially available ELISA kit (Elabscience, Biotechnology Inc. 2018).
Statistical analysis
SPSS (version 22.0) was used to carry out the analysis. Results were expressed as mean (standard error of the mean) of study variables. Independent sample t test was used to compare means of variables between two groups where P<0.05 was considered statistically significant.
RESULTS
Working memory
As shown in table 1, the mean values of escape latency in experimental rats were significantly (P<0.001) higher compared to those of control rats in all the trials in all the experimental days.
Reference memory
As shown in table 2, the mean values of escape latency in experimental rats were significantly (P<0.001) higher to control rats in all the days in all the experimental days (day 38 to day 43). Moreover, the mean value of target crossings was significantly (P<0.01) lower in experimental rats on day 44.
Hippocampal catalase and glutathione peroxidase
As shown in table 3, the mean value of catales and glutathione peroxidase were significantly (P<0.001) lower in experimental rats compared to that of control rats on day 50.
DISCUSSION
We report here that SCS causes working and reference memory impairment along with a decline in catalase and glutathione peroxidase in the experimental rats. Similar findings of escape latency and target crossing17, 23, 26 as well as decrease of hippocampal catalase and glutathione peroxidase were reported27, 28 after exposure to SCS.
It is reported that, the whole-body exposure of SCS, which contain various of oxidants29 including nicotine, carbon monoxide, heavy metals, hydrogen cyanide and polycyclic aromatic hydrocarbons4 are capable of initiating or promoting lipid peroxidation in any tissue of body, including hippocampus11, 29, 30. Even prolonged exposure of mild SCS caused decrease in hippocampal neurogenesis.
It is well known that neuronal connectivity is strictly dependent on synaptic strength and synaptic plasticity, which are basis for learning as well as working and reference memory. Here, persistent firing of pyramidal neurons and synaptic plasticity are the leading phenomenon especially at the hippocampal level, where it is essential for acquisition and retrieval of various types of memory. The persistent strengthening of the synapses following high levels of stimulation is called long-term potentiation and represents the main mechanism for learning and memory formation. This long-term potentiation causes consolidation of memory storage which persists for several hours or days. If the hippocampus is lesioned during acquisition or consolidation, working and reference memories are impaired.30
We acknowledge that the current study had several limitations. The memory performance test was manually operated, and only two variables each were assessed for antioxidant enzymes and reference retrieval.
Conclusion
SCS causes working as well as reference memory impairment and decrease in hippocampal antioxidant enzyme levels in male Long-Evans rats. This study has potential to support the tobacco control initiatives, in Bangladesh and elsewhere.
Acknowledgments
Special thanks to the chairman and other members of Department of Physiology, BSMMU.
Author contributions
Conception and design: AB, MSIP, AAT and SS. Acquisition, analysis, and interpretation of data: AB, MSIP, and AAT. Manuscript drafting and revising it critically: AB, MSIP, AAT and SS. Approval of the final version of the manuscript: AB, MSIP, AAT, and SS. Guarantor of accuracy and integrity of the work: AB and MSIP.
Funding
The study was funded by BSMMU.
Conflicts of interest
We do not have any conflict of interest.
Ethical approval
We obtained ethical approval from the Institutional Review Board of BSMMU, bearing memo number BSMMU/2022/8694, dated 31 August 2022.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author
References
1. Badamasi IM, Lawal IU. Post chronic cigarette smoke exposure & assessment of learning, memory and asymptomatic level of performance in Wistar rats. Bajopas. 2015; 8(2):46-57. DOI: http://dx.doi.org/10.4314/bajopas.v8i2.10
2. Pieraccini G, Furlanetto S, Orlandini S, Bartolucci G, Giannini I, Pinzauti S, Moneti G. Identification and determination of mainstream and sidestream smoke components in different brands and types of cigarettes by means of solid-phase microextraction-gas chromatography-mass spectrometry. J Chromatogr A. 2008 Feb 8;1180(1-2):138-150. DOI: https://doi.org/10.1016/j.chroma.2007.12.029
3. Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tobacco control. 2005; 14(6): 396-404. DOI: http://dx.doi.org/10.1136/tc.2005.011288
4. Csabai D, Csekő K, Szaiff L, Varga Z, Miseta A, Helyes Z, Czéh B. Low intensity, long term exposure to tobacco smoke inhibits hippocampal neurogenesis in adult mice. Behav Brain Res. 2016 Apr 1;302:44-52. DOI: https://doi.org/10.1016/j.bbr.2016.01.022
5. He Y, Lam T H, Jiang B, Wang J, Sai X, Fan L, Li X, Qin Y, Hu FB. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation. 2008; 118(15):1535–1540. DOI: https://doi.org/10.1161/CIRCULATIONAHA.108.784801
6. Nakata A, Takahashi M, Ikeda T, Hojou M, Nigam JA, Swanson NG. Active and passive smoking and depression among Japanese workers. Prev Med. 2008; 46(5):451-456. DOI: https://doi.org/10.1016/j.ypmed.2008.01.024
7. Sabanayagam C, Shankar A. The association between active smoking, smokeless tobacco, second-hand smoke exposure and insufficient sleep. Sleep Med. 2011; 12(1):7-11. DOI: https://doi.org/10.1016/j.sleep.2010.09.002
8. Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW, Mak JC, Chang RC. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS One. 2012;7(5):e36752. DOI: https://doi.org/10.1371/journal.pone.0036752
9. Khorasanchi Z, Bahrami A, Avan A, Jaberi N, Rezaey M, Bahrami-Taghanaki H, Ferns GA, Ghayour-Mobarhan M. Passive smoking is associated with cognitive and emotional impairment in adolescent girls. J Gen Psychol. 2019; 146(1): 68-78. DOI: https://doi.org/10.1080/00221309.2018.1535485
10. Delibas N, Ozcankaya R, Altuntas I, Sutcu R. Effect of cigarette smoke on lipid peroxidation, anti-oxidant enzymes and NMDA receptor subunits 2A and 2B concentration in rat hippocampus. Cell Biochem Funct.2003; 21(1):69-73. DOI: https://doi.org/10.1002/cbf.990
11. Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001; 40(8):959-975. DOI: https://doi.org/10.1016/S0028-3908(01)00019-3
12. Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus. 2010; 20(5):637-645. DOI: https://doi.org/10.1002/hipo.20657
13. Ke Q, Yang L, Cui Q, Diano W, Zhang Y, Xu M, He B. Ciprofibrate attenuates airway remodeling in cigarette smoke-exposed rats. Respir Physiol Neurobiol. 2020; 271:e103290. DOI: https://doi.org/10.1016/j.resp.2019.103290
14. DiGiacomo SI, Jazayeri MA, Barua RS, Ambrose JA. Environmental tobacco smoke and cardiovascular disease. IJERPH. 2018; 16(1):e96. DOI: https://doi.org/10.3390/ijerph16010096
15. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1(2):848-858. DOI: https://doi.org/10.1038/nprot.2006.116
16. Wang P, Wang Y, Zhang Q, Zhang H, Li Z, Liu X, Kaur L, Kumar M. Amelioration of cognitive deficits by Spirulina platensis in L-methionine induced rat model of vascular dementia. Phacog Mag. 2020; 16(68):133-141. DOI: http://doi.org/10.4103/pm.pm_438_19
17. Wren-Dail MA, Dauchy RT, Ooms TG, Baker KC, Blask DE, Hill SM, Dupepe LM, Bohmjr RP. Effects of colored enrichment devices on circadian metabolism and physiology in male Sprague-Dawley rats. Comp Med. 2016; 66(5):384-391. URL: https://www.ingentaconnect.com/content/aalas/cm/2016/00000066/00000005/art00004#
18. Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab. 2018; 7:161-170. DOI: https://doi.org/10.1016/j.molmet.2017.10.009
19. Islam KMN, Rahman ASMH, Al-Mahmud KA. Manual for care and use of laboratory animals.icddrb. 2001; Bangladesh. URL: https://labservices.icddrb.org/animal-resources-facility
20. Deboer T. Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other’s functioning? Neurobiol Sleep Circadian Rhythm. 2018; 5:68-77. DOI: https://doi.org/10.1016/j.nbscr.2018.02.003
21. Ypsilantis P, Politou M, Anagnostopoulos C, Kortsaris A, Simopoulos C. A rat model of cigarette smoke abuse liability. Comp Med. 2012; 62(5):395-399. URL: https://www.ingentaconnect.com/content/aalas/cm/2012/00000062/00000005/art00006
22. Imam MI, Adamu A, Muhammad UA, Yusha’u Y. Nigella sativa (black seed) extract improves spatial learning ability in albino mice. Bayero J Pure Appl Sci. 2017; 10(2):111-114. DOI: http://doi.org/10.4314/bajopas.v10i2.19
23. Topuz RD, Gunduz O, Tastekin E, Karadag CH. Effects of hippocampal histone acetylation and HDAC inhibition on spatial learning and memory in the Morris water maze in rats. Fundam Clin Pharmacol. 2020; 34(2):222-228. DOI: https://doi.org/10.1111/fcp.12512
24. Paul CA, Beltz B, Berger-Sweeney J. Dissection of rat brains. CSH protocols. 2008; e4803. DOI: https://doi.org/10.1101/pdb.prot4803
25. Sultan FA. Dissection of different areas from mouse hippocampus. Bio protocol. 2013; 3(21):e955. DOI: https://doi.org/10.21769/BioProtoc.955
26. Badamasi IM, Lawal IU. Post chronic cigarette smoke exposure & assessment of learning, memory and asymptomatic level of performance in Wistar rats. Bajopas. 2015; 8(2):46-57. DOI: https://doi.org/10.4314/bajopas.v8i2-10
27. Ramesh T, Sureka C, Bhuvana S, Begum VH. Oxidative stress in the brain of cigarette smoke-induced noxiousness: neuroprotective role of Sesbania grandiflora. Metab Brain Dis. 2015; 30(2):573-582. DOI: https://doi.org/10.1007/s11011-014-9614-4
28. Alzoubi KH, Khabour OF, Alharahshah EA, Alhashimi FH, Shihadeh A, Eissenberg T. The effect of waterpipe tobacco smoke exposure on learning and memory functions in the rat model. J Mol Neurosci. 2015; 57:249-256. DOI: https://doi.org/10.1007/s12031-015-0613-7
29. Luchese C, Pinton S, Nogueira CW. Brain and lungs of rats are differently affected by cigarette smoke exposure: antioxidant effect of an organoselenium compound. Pharmacol Res. 2009; 59(3):194-201. DOI: https://doi.org/10.1016/j.phrs.2008.11.006
30. Bisaz R, Travaglia A, Alberini CM. The neurobiological bases of memory formation: from physiological conditions to psychopathology. Psychopathology. 2014; 47(6):347-356. DOI: https://doi.org/10.1159/000363702
|
|
|
||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||
(c) 2024 The Authors. Published by BSMMU Journal