|
Bangabandhu Sheikh Mujib Medical University Journal Volume 17, Issue 1, March 2024
CASE REPORT 3D printing technology in the management of carpal tunnel syndrome:
A care report
Md.
Israt Hasan1
1Department of Physical Medicine and Rehabilitation, Kurmitola General Hospital, Dhaka, Bangladesh. 2Department of Robotics and Mechatronics Engineering, University of Dhaka, Dhaka, Bangladesh. 3Department of Physical Medicine and Rehabilitation, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh. 4Department of Orthopedic Surgery, Kurmitola General Hospital, Dhaka, Bangladesh.
DOI: https://doi.org/10.3329/bsmmuj.v17i1.71690 Received: 29 Feb 2024; Revised version received: 20 Mar 2024; Accepted: 25 Mar 2024 Published online: 30 Mar 2024 Responsible
Editor: M Mostafa Zaman |
ABSTRACT
A 35-year-old individual with carpal tunnel syndrome presented with tingling and numbness in the left thumb, index, and middle finger. A 3D printed CTS splint was crafted to immobilize the affected wrist joint, aiding pharmacotherapy. At six weeks, evaluations included the Boston Carpal Tunnel Questionnaire (BCTQ), Visual Analogue Scale (VAS) for pain, and Evaluation of Satisfaction with assistive Technology (QUEST) version 2.0.9. Substantial improvements were observed in Bangla-BCTQ scores (symptom severity scale: 3.68 vs. 1.27; functional status scale: 2.74 vs. 1.31), VAS (70 vs. 30), and QUEST scores. 3D printing technology may contribute to better personalized musculoskeletal care enhancing quality of life.
INTRODUCTION
3D printed orthoses are increasingly recognized as a viable choice within the realm of Physical Medicine and Rehabilitation for creating traditional casts and orthoses. Orthoses are rigid or semi-rigid devices used to support, restrict, mobilize, or immobilize injured body segments, aiding healing and function improvement. They can be custom-made, prefabricated, or custom-fitted by clinicians. Splints or braces, synonymous with orthoses, can be made from plaster, fiberglass, or 3D printed materials.1 Several studies have demonstrated the efficacy of wrist splinting in relieving the symptoms of Carpal tunnel syndrome however, the chosen angle of immobilization has varied.2
CASE DESCRIPTION
A 35-year-old woman presented to the Physical Medicine and Rehabilitation outpatient department at Bangabandhu Sheikh Mujib Medical University with tingling and numbness in left hand for three months, diagnosed clinically, and confirmed with electrophysiological findings as CTS.
CTS results from median nerve compression within the carpal tunnel, a common upper limb entrapment neuropathy.3, 4 Management focuses on alleviating pressure on the median nerve through conservative or minimally invasive approaches. Mild to moderate symptoms are typically addressed using wrist splinting, carpal canal steroid injections, exercises, yoga, therapeutic ultrasound, ergonomic adjustments, and oral vitamin medication.5, 6 Along with conventional pharmacological therapy, she was advised a 3D printed carpal tunnel splint for 6 weeks. Advantages of 3D printed splints compared to conventional ones include easier modification and faster fabrication.7 The 3D printing technique makes it possible for physicians to easily create individual-tailored products.
LEARNING POINTS
1. Custom 3D printed splints are effective for carpal tunnel syndrome treatment.
2. The splint, combined with medication, improved symptoms, reduced pain, and increased patient satisfaction.
3. Collaboration among patients, clinicians, and engineers enhances personalized musculoskeletal care, improving quality of life.
CASE MANAGEMENT
Physiatrists from Bangabandhu Sheikh Mujib Medical University (BSMMU) and Kurmitola General Hospital, Dhaka collaborated with engineers from the Department of Robotics and Mechatronics Engineering (RME), University of Dhaka to develop a solution. The required CTS splint is needed to immobilize the wrist joint of the patient for 6 weeks. It was deemed that a personalized, 3D printed CTS splint was the best way to meet these criteria. The entire process from solution conception, through design, and manufacture was done in the RME Lab of Dhaka University with point-of-care manufacturing facilities and clinical testing was completed within the BSMMU campus. This process involved.
· The lightweight and durable material used in 3D printed CTS splint provides comfortable supports for the patient’s wrist. After careful consideration of two available 3D printing material—poly lactic acid (PLA) and acrylonitrile butadiene styrene (ABS), PLA was selected because of its durability (Table 1).
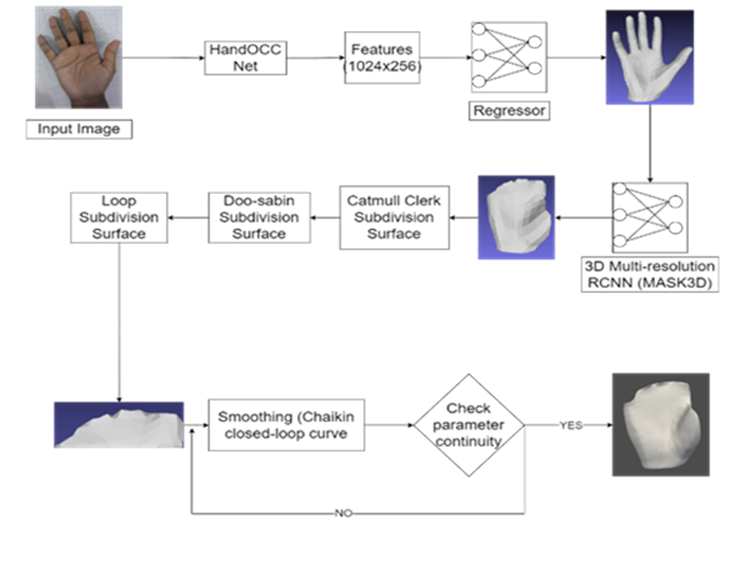
· Patient's hand was scanned using a setup with various sensors.
· Red green blue images were captured by the setup.
· A deep learning model was implemented to extract two separate scaled 3D meshes of palmar and dorsal parts of the hand from the RGB images.
· The 3D meshes were further processed to obtain a 3D printable format.
· The separate parts of the orthosis were printed using an fused deposition modeling 3D printer.
· The parts were attached using adjustable straps to create the final custom-made orthosis.
The 3D printed CTS was evaluated for fit and efficacy and underwent minor adjustments based on patient and physician’s feedback. The patient completed the validated Bangla version of the Boston Carpal Tunnel Questionnaire (Bangla-BCTQ)8 and Visual Analogue Scale (VAS) and reviewed by two physiatrists and an orthopedic surgeon to measure the impact of this device on the patient's symptom severity and functional status. Patient’s satisfaction with the splint was assessed by Quebec User Evaluation of Satisfaction with assistive Technology (QUEST) version 2.0.9 Outcome measures were completed before, and after three and six weeks of device use.
The chosen outcome measures, including the BCTQ, VAS, and QUEST version 2.0, were selected for their established validity and relevance in assessing symptom severity, pain intensity, and patient satisfaction, respectively, providing a comprehensive evaluation of the patient experience in managing carpal tunnel syndrome with the personalized, 3D-printed splint.8, 9, 10
After six weeks of device use, the patient had not experienced any kind of tingling or numbness, whilst demonstrating clinically significant improvements in Bangla-BCTQ and VAS scores (Table 2). The BCTQ scores BCTQ scores for the 3D printed CTS splint at baseline were 3.68 (SSS), 2.74 (FSS), and 70 (VAS), and in the 6th week were 1.27 (SSS), 1.31 (FSS), and 30 (VAS). Bangla version of The Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST) version 2.0 was used to evaluate patients' satisfaction with the 3D printed orthosis.10 QUEST is a self-report or interview-based scale designed to evaluate a person's satisfaction with a wide range of assistive technology. The calculated total QUEST score was 4. The results suggest that 3D printed orthosis is a feasible option for CTS patients.
DISCUSSION
Decreased VAS scores suggest 3D printed orthoses effectively alleviate CTS symptoms, enhancing patients' quality of life. Improved BCTQ scores affirm the efficacy of customized orthoses, enhancing daily activities by providing tailored support and stability. Positive feedback on customized orthosis comfort and usability, reflected by high QUEST scores, underscores its potential to enhance patient satisfaction.
This marks the inaugural implementation of a custom-made 3D printed orthosis for CTS patients in Bangladesh. Collaborative point-of-care design and manufacturing of personalized devices can significantly enhance clinical outcomes and quality of life for individuals with musculoskeletal conditions, underscoring the untapped potential of 3D technologies in physical medicine and rehabilitation.
While this case study offers valuable insights into personalized splinting for CTS, limitations exist. The single-case design limits generalizability, warranting further research with larger cohorts. Additionally, outcome measures, while standard, may not fully capture symptom complexity. Potential biases, including placebo effects and concurrent treatments, require consideration in future studies. Moreover, while collaborative efforts enhance innovation, scalability and accessibility challenges persist. Addressing cost-effectiveness, regulatory hurdles, and technological constraints is crucial for widespread adoption. These limitations underscore the need for a robust investigation to validate findings and optimize personalized musculoskeletal care approaches.
Acknowledgments
We thank the members of the Department of Robotics and Mechatronics Engineering, University of Dhaka, for their kind support.
Author contributions
Conception and design: MIH. Acquisition, analysis, and interpretation of data: MT, SMA, SAD. Manuscript drafting and revising it critically: MIH, TM, MT. Approval of the final version of the manuscript: MIH, SMA. Guarantor of accuracy and integrity of the work: MIH.
Funding
Ministry of Science and Technology, Government of the People’s Republic of Bangladesh.
Conflicts of interest
We do not have any conflict of interest.
Ethical approval
Ethical approval was not sought because this is a case report. However, consent was obtained from the patient to prepare this manuscript.
Data availability statement sharing
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
1. Schwartz DA, Schofield KA. Utilization of 3D printed orthoses for musculoskeletal conditions of the upper extremity: A systematic review. J Hand Ther. 2023 Jan-Mar;36(1):166-178. DOI: https://doi.org/10.1016/j.jht.2021.10.005
2. Burke DT, Burke MM, Stewart GW, Cambré A. Splinting for carpal tunnel syndrome: in search of the optimal angle. Archives of physical medicine and rehabilitation. 1994 Nov 1;75(11):1241-1244.DOI: https://doi.org/10.1016/0003-9993(94)90012-4
3. Hasan MI, Emran M, Newaz F, Atiquzzaman M, Morshed T, Banik A, Sadeque AZ, Sharmin S. Physiatric Management of Carpal Tunnel Syndrome. Khwaja Yunus Ali Medical College Journal. 2020 Mar. 1;10(4):206-210. DOI: https://doi.org/10.3329/kyamcj.v10i4.45721
4. Hasan MI, Ahmed SM. Intralesional steroid and ultrasound therapy in patients with carpal tunnel syndrome. Bangabandhu Sheikh Mujib Medical Univ J. 2019 Dec. 23;12(4):177-181. DOI: https://doi.org/10.3329/bsmmuj.v12i4.44051
5. Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J. 2008 Jan;77(1):6-17. PMID: 18269111
6. Muller M, Tsui D, Schnurr R, Biddulph-Deisroth L, Hard J, MacDermid JC. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: A systematic review. J Hand Ther. 2004; 17: 210-228. DOI: https://doi.org/10.1197/j.jht.2004.02.009
7. Chae DS, Kim DH, Kang KY, Kim DY, Park SW, Park SJ, Kim JH. The functional effect of 3D-printing individualized orthosis for patients with peripheral nerve injuries: Three case reports. Medicine. 2020 Apr;99(16). DOI: https://doi.org/10.1097/md.0000000000019791
8. Hasan MI, Emran M, Atiquzzaman M, Morshed T, Ahmed SM, Hasan AM, Chowdhury ZR. Bangla Version of the Boston Carpal Tunnel Questionnaire: Translation, Cross-Cultural Adaptation, Validation and Reliability Assessment. Khwaja Yunus Ali Medical College Journal. 2022 Jun. 5;13(1):24-31. DOI: https://doi.org/10.3329/kyamcj.v13i1.59877
9. Demers L, Weiss-Lambrou R, Ska B. Development of the Quebec user evaluation of satisfaction with assistive technology (QUEST). Assistive technology. 1996 Jun 30;8(1):3-13. DOI: http://dx.doi.org/10.1080/10400435.1996.10132268
10. Hasan MI, Ahmed SM. Bangla version of Quebec User Evaluation of Satisfaction with assistive Technology (QUEST version 2.0). 2023. DOI: http://dx.doi.org/10.13140/RG.2.2.23236.40321
|
|||||||||||||||||||||
|
||||||||||||||||||||||
|
FIGURE 1 Deep learning algorithm to extract 3D model |
(c) 2024 The Authors. Published by BSMMU Journal