Barraquer–Simons syndrome: A case report
Authors
- Mita DuttaDepartment of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
- Munira Afroz SiddikaDepartment of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
- Mohammad Aminul IslamDepartment of Endocrinology, National Academy for Planning and Development, Dhaka, Bangladesh
- Hurjahan BanuDepartment of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
DOI:
Keywords
Downloads
Correspondence
Publication history
Handling editor
Reviewers
Funding
Ethical approval
Trial registration number
Copyright
Published by Bangabandhu Sheikh
Mujib Medical University
Categories | Number (%) |
Sex |
|
Male | 36 (60.0) |
Female | 24 (40.0) |
Age in yearsa | 8.8 (4.2) |
Education |
|
Pre-school | 20 (33.3) |
Elementary school | 24 (40.0) |
Junior high school | 16 (26.7) |
Cancer diagnoses |
|
Acute lymphoblastic leukemia | 33 (55) |
Retinoblastoma | 5 (8.3) |
Acute myeloid leukemia | 4 (6.7) |
Non-Hodgkins lymphoma | 4 (6.7) |
Osteosarcoma | 3 (5) |
Hepatoblastoma | 2 (3.3) |
Lymphoma | 2 (3.3) |
Neuroblastoma | 2 (3.3) |
Medulloblastoma | 1 (1.7) |
Neurofibroma | 1 (1.7) |
Ovarian tumour | 1 (1.7) |
Pancreatic cancer | 1 (1.7) |
Rhabdomyosarcoma | 1 (1.7) |
aMean (standard deviation) | |
Categories | Number (%) |
Sex |
|
Male | 36 (60.0) |
Female | 24 (40.0) |
Age in yearsa | 8.8 (4.2) |
Education |
|
Pre-school | 20 (33.3) |
Elementary school | 24 (40.0) |
Junior high school | 16 (26.7) |
Cancer diagnoses |
|
Acute lymphoblastic leukemia | 33 (55) |
Retinoblastoma | 5 (8.3) |
Acute myeloid leukemia | 4 (6.7) |
Non-Hodgkins lymphoma | 4 (6.7) |
Osteosarcoma | 3 (5) |
Hepatoblastoma | 2 (3.3) |
Lymphoma | 2 (3.3) |
Neuroblastoma | 2 (3.3) |
Medulloblastoma | 1 (1.7) |
Neurofibroma | 1 (1.7) |
Ovarian tumour | 1 (1.7) |
Pancreatic cancer | 1 (1.7) |
Rhabdomyosarcoma | 1 (1.7) |
aMean (standard deviation) | |
Pain level | Number (%) | P | ||
Pre | Post 1 | Post 2 | ||
Mean (SD)a pain score | 4.7 (1.9) | 2.7 (1.6) | 0.8 (1.1) | <0.001 |
Pain categories | ||||
No pain (0) | - | 1 (1.7) | 31 (51.7) | <0.001 |
Mild pain (1-3) | 15 (25.0) | 43 (70.0) | 27 (45.0) | |
Moderete pain (4-6) | 37 (61.7) | 15 (25.0) | 2 (3.3) | |
Severe pain (7-10) | 8 (13.3) | 2 (3.3) | - | |
aPain scores according to the visual analogue scale ranging from 0 to 10; SD indicates standard deviation | ||||




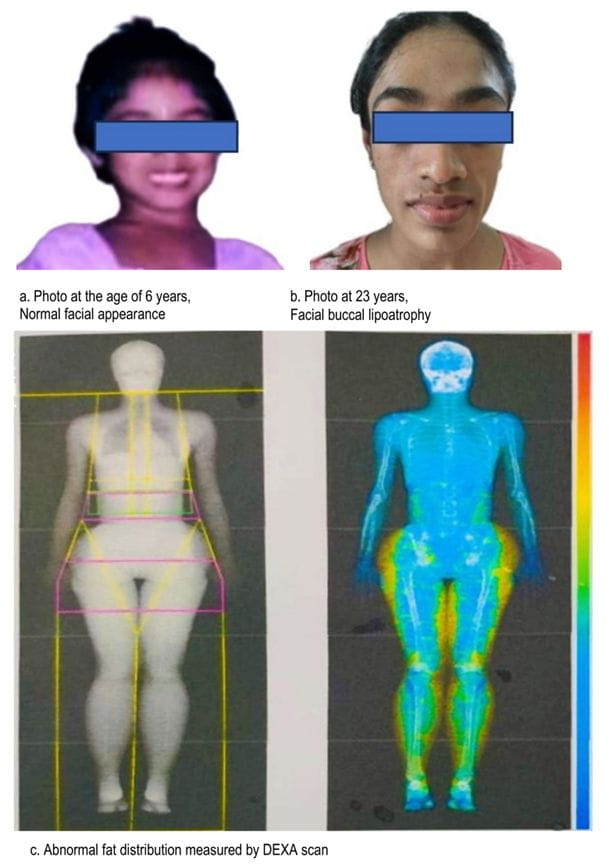
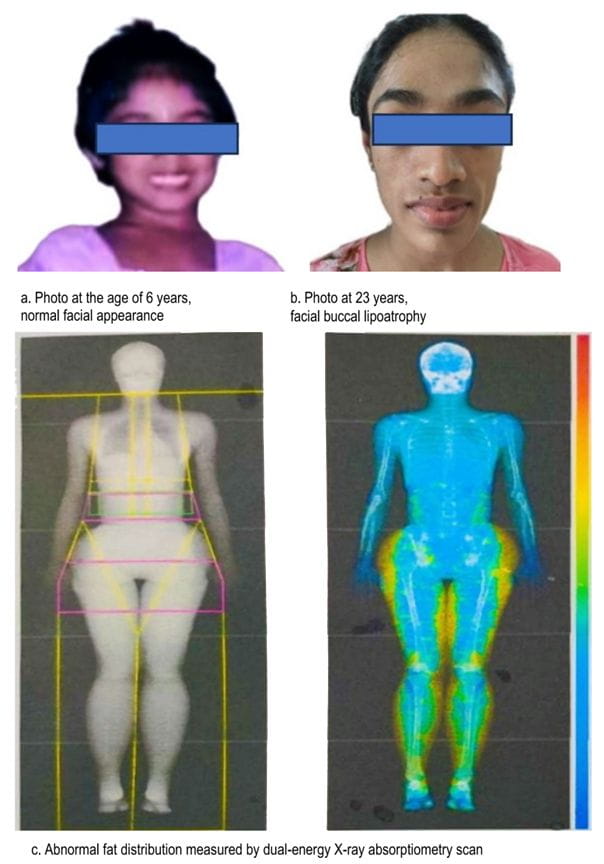
Yellow color represents increased fat mass in lower body.
BSS has a female preponderance, with a median age of presentation at 25 years [4]. This disease is believed to have originated from unknown causes, but it exhibits implications of viral illnesses, such as chickenpox and measles, as well as autoimmune associations with systemic lupus erythematosus (SLE), vasculitis, and other conditions. Although 80% of cases are associated with autoimmune diseases, most cases can develop them many years after the initial diagnosis of lipodystrophy (systemic lupus erythematosus, 4–37 years; vasculitis, 17–28 years) [4]. BSS is atypical from other lipodystrophies. Although the predominant abnormality is hypertriglyceridemia, hypercholesterolemia can be present in a minority of cases [4].

