Comparison of disability levels between haemorrhagic and ischaemic stroke in the sub-acute phase: A cross-sectional study
Authors
- Md. Nurul Hoque MiahDepartment of Physical Medicine and Rehabilitation, Sher-E-Bangla Medical College and Hospital, Barishal, Bangladesh
- Md. Israt HasanDepartment of Physical Medicine and Rehabilitation, Sher-E-Bangla Medical College and Hospital, Barishal, Bangladesh
- Moinuddin Hossain KhanDepartment of Physical Medicine and Rehabilitation, National Institute of Traumatology and Orthopedic Rehabilitation, Dhaka, Bangladesh
DOI:
Keywords
Downloads
Correspondence
Publication history
Responsible editor
Reviewers
Funding
Ethical approval
Trial registration number
Copyright
Published by Bangladesh Medical University (former Bangabandhu Sheikh Mujib Medical University).
Background: Stroke remains a leading cause of disability worldwide, with hemiplegia being a common consequence. The Barthel Index (BI) is a widely used tool for assessing disability in activities of daily living (ADL). This study aimed to evaluate the level of disability among patients with sub-acute hemiplegic stroke and compare disability levels between ischaemic and haemorrhagic stroke within 3 weeks of onset in an acute rehabilitation setting.
Methods: A cross-sectional study was conducted at Sher-E-Bangla Medical College and Hospital in the Barishal division of Bangladesh, from October 2022 to March 2023. Seventy-five patients aged 20–85 years, experiencing a first-ever stroke with hemiplegia, were assessed using the BI. Patients with subarachnoid haemorrhage, recurrent stroke, or severe comorbidities were excluded. BI scores and dependency levels were expressed in mean and standard deviation and compared between groups using Student’s t tests, with statistical significance set at P <0.05.
Results: The mean (standard deviation) BI scores were significantly higher (P <0.001) in ischaemic stroke patients, 62.0 (20.8), compared to haemorrhagic stroke patients, 24.6 (21.3). The ischaemic stroke patients predominantly exhibited severe dependency (64.1%), while haemorrhagic stroke patients showed total dependency (52.8%). Bathing, bladder control, and stair climbing were the most affected ADL domains in both groups. Hypertension was the most common risk factor (62.7%), followed by diabetes mellitus (37.3%).
Conclusion: Haemorrhagic stroke patients exhibit greater disability than ischaemic stroke patients in the acute rehabilitation phase. These findings underscore the need for tailored rehabilitation strategies to address severe dependency, particularly in haemorrhagic stroke survivors.
Stroke remains a leading cause of adult disability worldwide. In 2019, there were approximately 143 million disability-adjusted life years lost due to stroke, with sub-Saharan and South Asian regions bearing a disproportionate burden [1]. In Bangladesh, the incidence and prevalence of stroke continue to rise, estimated at approximately 11 per 1000 population, with ischaemic strokes accounting for two-thirds of cases [2, 3]. Hemiplegia, partial or complete paralysis of one side of the body, is one of the most prevalent and disabling sequelae of stroke, affecting up to 80% of survivors early on [3].
The immediate period following a stroke, particularly within the first few weeks, is critical for functional recovery. Comprehensive rehabilitation interventions initiated during this sub-acute phase have been shown to significantly improve functional independence and reduce long-term disability. Accurate and reliable assessment of disability levels during this period is essential for tailoring rehabilitation programs to individual patient needs and monitoring their progress [4].
The Barthel Index (BI) is a widely recognized and validated tool used to assess functional independence in performing activities of daily living (ADL) [5]. It provides a quantitative measure of disability by evaluating a patient's ability to perform ten basic ADLs, including feeding, bathing, grooming, dressing, bowel and bladder control, toilet use, transfers from bed to chair and back, mobility on level surfaces, and stairs [6]. The BI is known for its ease of administration, reliability, and sensitivity to changes in functional status, making it a valuable instrument in both clinical practice and research settings for stroke rehabilitation [7].
Despite the recognized burden of stroke and the importance of early disability assessment, comparative data on the levels of disability between ischaemic and haemorrhagic strokes within three weeks of onset remain limited, particularly in the context of Bangladesh. This study aimed to evaluate the level of disability among patients with sub-acute hemiplegic stroke in a tertiary care hospital in Bangladesh and compare the disability levels between those with ischaemic and haemorrhagic stroke in an acute rehabilitation setting.
Study design and participants
This cross-sectional study was conducted in the Department of Physical Medicine and Rehabilitation at Sher-E-Bangla Medical College Hospital, Barishal, Bangladesh, from October 2022 to March 2023. This study consecutively enrolled patients with a first-ever stroke with hemiplegia. Inclusion criteria were age between 20–85 years, assessed within three weeks of stroke onset and within 48 hours of admission, and diagnosis of hemiplegia confirmed by clinical examination and computed tomography (CT) scan of the brain. Patient were excluded if they had subarachnoid haemorrhage, a history of recurrent stroke, severe comorbidities (e.g., persistent unconsciousness, recent myocardial infarction). A total of 75 patients meeting the eligibility criteria were included in the analysis. Ischaemic and haemorrhagic stroke types were classified based on CT scan findings.
Instruments and data collection
Data were collected using a structured case record form that included sociodemographic variables (age, sex, education, occupation, residence), stroke characteristics (side of hemiplegia, handedness etc.), and risk factors (e.g., hypertension and diabetes).
Disability was assessed by trained postgraduate doctors familiar with standardized Barthel Index (BI) administration within 48 hours of admission, which evaluates 10 activities of daily living (ADL) domains (feeding, bathing, grooming, dressing, bowel, bladder, toilet use, transfers, mobility and stairs) with a total score ranging from 0 (total dependency) to 100 (full independence). BI scores were categorised as: 0–20 (total dependency), 21–60 (severe dependency), 61–90 (moderate dependency), 91–99 (slight dependency), and 100 (complete independence) [5]. Assessments were performed at admission (or specify timing) by trained postgraduate doctors using standardized instructions, through direct observation and patient self-report.
Ethical considerations
This study was conducted following strict adherence to ethical principles outlined in the Declaration of Helsinki. Informed written consent was obtained from all participants or their legally authorised representatives after providing clear explanations about the study objectives, procedures, potential risks, and benefits. Participants were assured that their involvement was voluntary and that they could withdraw at any point without affecting their standard care. Confidentiality and anonymity of all personal and clinical data were strictly maintained. No invasive procedures or interventions were carried out as part of the study. Only routine clinical assessments and non-invasive disability evaluations were included. No financial or material inducements were provided for participation.
Statistical analysis
Data were analysed using SPSS version 20. Descriptive statistics, including frequencies, percentages, means, and standard deviations, were used to summarise demographic and clinical variables. There were no missing data for primary outcome variables, and data distribution was assessed prior to analysis and deemed suitable for parametric testing. An independent-sample Student’s t test was used to compare mean BI scores and domain-specific ADL scores between ischaemic and haemorrhagic stroke groups. Categorical variables, including levels of dependency, were compared using the chi-square test or Fisher’s exact test, as appropriate. A P of < 0.05 was considered statistically significant.


Demographic characteristics
Of the 75 patients, 50 (66.7%) were men and 25 (33.3%) were women (between-group P = 0.99). The overall mean (standard deviation) age was 58.0 (13.5) years, with no significant difference between ischaemic 60.0 (12.8) years and haemorrhagic stroke patients 56.0 (14.9) years. Haemorrhagic stroke were more frequent in older adults (60-85 years) the compared with the ischaemic group (41.7% vs. 20.5%, P = 0.020) compared to younger adults (20-59 years). The distribution of risk factors did not differ significantly between stroke subtypes (Table 1).
Table 1 Background and clinical characteristics of the study participants with stroke (n=75)
Variables | Overall | Ischaemic | Haemorrhagic | P |
n=75 | n=39 | n=36 |
| |
Age group |
|
|
|
|
20-59 | 52 (69.3) | 31 (79.5) | 21 (58.3) | 0.02 |
60-85 | 23 (30.7) | 8 (20.5) | 15 (41.7) |
|
Sex |
|
|
|
|
Men | 50 (66.7) | 26 (66.7) | 24 (66.7) | 0.99 |
Women | 25 (33.3) | 13 (33.3) | 12 (33.3) |
|
Side of hemiplegia |
|
|
|
|
Right | 44 (58.7) | 24 (61.5) | 20 (55.6) | 0.59 |
Left | 31 (41.3) | 15 (38.5) | 16 (44.4) |
|
Clinical impairments |
|
|
|
|
Speech abnormalities | 47 (62.7) | 23 (59.0) | 24 (66.7) | 0.49 |
Spasticity | 39 (52.0) | 15 (38.5) | 24 (66.7) | 0.02 |
Dysphagia | 22 (29.3) | 9 (23.1) | 16 (44.4) | 0.05 |
Bowel/bladder incontinence | 5 (6.7) | 4 (10.3) | 1 (2.8) | 0.20a |
Risk factors |
|
|
|
|
Hypertension | 47 (62.7) | 25 (64.1) | 22 (61.1) | 0.80 |
Diabetes mellitus | 28 (37.3) | 17 (43.6) | 11 (30.6) | 0.24 |
Smoking | 23 (30.7) | 13 (33.3) | 10 (27.8) | 0.60 |
Family history | 15 (20.0) | 9 (23.1) | 6 (16.7) | 0.49 |
Barthel Index score group | ||||
Total dependency (0–20) | 19 (25.3) | 0 (0) | 19 (52.8) | <0.01a |
Severe dependency (21–60) | 41 (57.0) | 25 (64.1) | 17 (47.2) |
|
Moderate dependency (61–90) | 10 (13.3) | 10 (25.6) | 0 (0) |
|
Slight dependency (91–99) | 0 (0) | 0 (0) | 0 (0) |
|
All are number (%); a Fisher’s exact test | ||||
Clinical impairments and risk factors
All participants were right-handed. Right-sided hemiplegia was observed in 44 patients (58.7%), with no difference between stroke subtypes. Within three weeks of stroke onset, common clinical impairments included speech abnormalities, spasticity, and dysphagia. Speech abnormalities were present in 62.7% of patients overall (59.0% ischaemic vs. 66.7% haemorrhagic). Spasticity was significantly more frequent in haemorrhagic stroke patients than in ischaemic stroke patients (66.7% vs. 38.5%, P = 0.02). Dysphagia was also more prevalent in haemorrhagic stroke (44.4%) compared with ischaemic stroke (23.1%). Bowel and bladder incontinence was uncommon overall and occurred in a small proportion of patients. Hypertension was the most prevalent vascular risk factor (62.7%), followed by diabetes mellitus (37.3%), smoking (30.7%), and positive family history (20.0%).
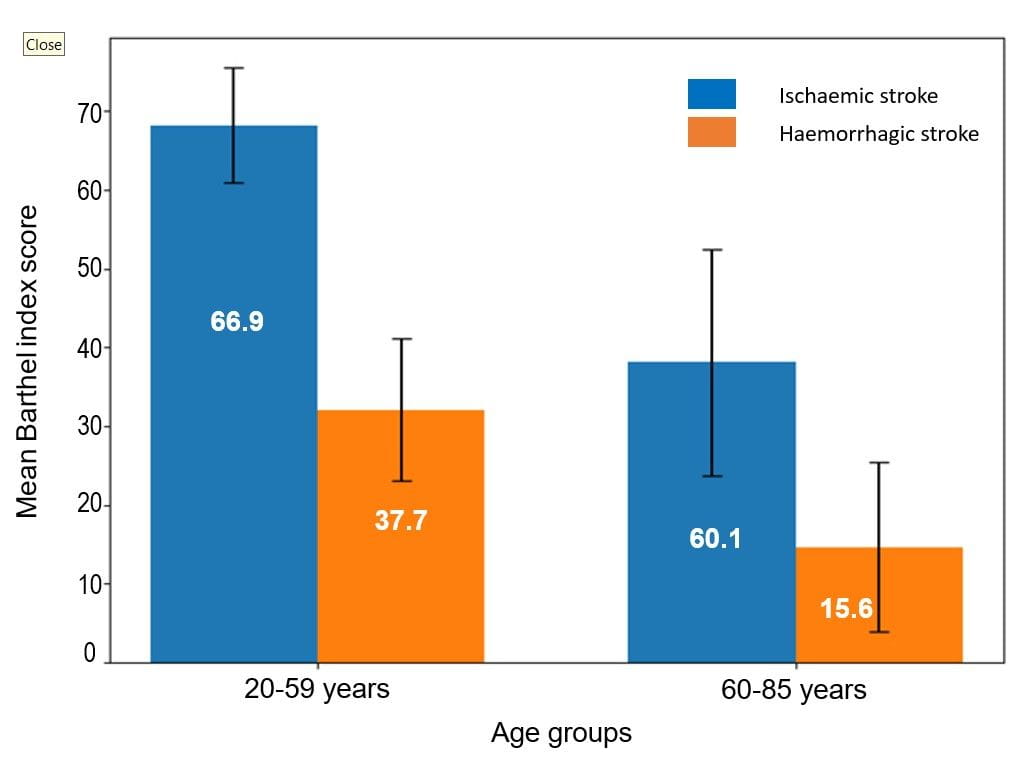
Figure 1 Mean Barthel Index (95% confidence interval) score by age group and stroke type (n=75)
Levels of dependency and ADL domains
Dependency levels differed significantly between groups (P < 0.001). In the ischaemic stroke group, 64.1% of patients had severe dependency (BI: 21–60) and 25.6% had moderate dependency (BI: 61–90), with no cases of total dependency. In contrast, 52.8% of haemorrhagic stroke patients had total dependency (BI: 0–20) and the remainder had severe dependency, with no moderate or slight dependency.
Disability outcomes
Mean Barthel Index (BI) scores were significantly higher in ischaemic stroke patients 62.0 (20.8) compared with haemorrhagic stroke patients 24.6 (21.3), indicating substantially greater functional independence in the ischaemic group (P < 0.001; 95% Confidence Interval for mean difference: 27.77–47.16). Age-stratified analysis demonstrated that older adults (60–85 years) had lower mean BI scores than younger adults (20–59 years) in both stroke subtypes. Across the age groups (20–59 and 60–85 years) patients with haemorrhagic stroke consistently exhibited markedly lower functional independence compared patients with ischaemic stroke, based on Barthel Index scores (Figure 1).
Table 2 Comparison of mean (standard deviation) Barthel activities of daily living (ADL) scoring of ischaemic and haemorrhagic stroke (n=75)
ADL scores | Ischaemic stroke | Haemorrhagic stroke | P |
Feeding score | 5.8 (2.9) | 4.2 (3.5) | 0.04 |
Bathing score | 1.2 (2.1) | 0 (0) | - |
Grooming score | 2.3 (2.5) | 1.8 (0.9) | 0.05 |
Dressing score | 6.3 (3.2) | 2.1 (2.5) | ˂0.001 |
Bowel score | 8.1 (2.5) | 1.5 (2.3) | ˂0.001 |
Bladder score | 9.0 (2.9) | 1.3 (3.0) | ˂0.001 |
Toilet use score | 6.2 (2.1) | 2.8 (3.3) | ˂0.001 |
Transfers score | 8.3 (4.0) | 3.6 (3.1) | ˂0.001 |
Mobility score | 10.0 (3.4) | 5.3 (4.0) | ˂0.001 |
Stair score | 5.1 (2.4) | 1.3 (2.2) | ˂0.001 |
Overall | 62.0 (20.8) | 24.6 (21.3) | ˂0.001 |
Across individual ADL domains, bathing, bladder control, and stair climbing were the most severely affected activities in both groups (Table 2). Haemorrhagic stroke patients demonstrated significantly lower scores across most ADL domains, particularly dressing, bowel and bladder control, toilet use, transfers, mobility, and stair climbing (P < 0.001), as well as feeding, bathing, and grooming (P < 0.05).
Variables | Frequency (%) |
Indication of colposcopy |
|
Visual inspection of the cervix with acetic acid positive | 200 (66.7) |
Abnormal pap test | 13 (4.3) |
Human papilloma virus DNA positive | 4 (1.3) |
Suspicious looking cervix | 14 (4.7) |
Others (per vaginal discharge, post-coital bleeding) | 69 (23.0) |
Histopathological diagnosis | |
Cervical Intraepithelial Neoplasia 1 | 193 (64.3) |
Cervical Intraepithelial Neoplasia 2 | 26 (8.7) |
Cervical Intraepithelial Neoplasia 3 | 32 (10.7) |
Invasive cervical cancer | 27 (9.0) |
Chronic cervicitis | 17 (5.6) |
Squamous metaplasia | 5 (1.7) |
Groups based on pre-test marks | Pretest | Posttest Marks (%) | Difference in pre and post-test marks (mean improvement) | P |
Didactic lecture classes | ||||
<50% | 36.6 (4.8) | 63.2 (9.4) | 26.6 | <0.001 |
≥50% | 52.8 (4.5) | 72.4 (14.9) | 19.6 | <0.001 |
Flipped classes | ||||
<50% | 36.9 (4.7) | 82.2 (10.8) | 45.4 | <0.001 |
≥50% | 52.8 (4.6) | 84.2 (10.3) | 31.4 | <0.001 |
Data presented as mean (standard deviation) | ||||
Background characteristics | Number (%) |
Age at presentation (weeks)a | 14.3 (9.2) |
Gestational age at birth (weeks)a | 37.5 (2.8) |
Birth weight (grams)a | 2,975.0 (825.0) |
Sex |
|
Male | 82 (41) |
Female | 118 (59) |
Affected side |
|
Right | 140 (70) |
Left | 54 (27) |
Bilateral | 6 (3) |
Delivery type |
|
Normal vaginal delivery | 152 (76) |
Instrumental delivery | 40 (20) |
Cesarean section | 8 (4) |
Place of delivery |
|
Home delivery by traditional birth attendant | 30 (15) |
Hospital delivery by midwife | 120 (60) |
Hospital delivery by doctor | 50 (25) |
Prolonged labor | 136 (68) |
Presentation |
|
Cephalic | 144 (72) |
Breech | 40 (20) |
Transverse | 16 (8) |
Shoulder dystocia | 136 (68) |
Maternal diabetes | 40 (20) |
Maternal age (years)a | 27.5 (6.8) |
Parity of mother |
|
Primipara | 156 (78) |
Multipara | 156 (78) |
aMean (standard deviation), all others are n (%) | |
Background characteristics | Number (%) |
Age at presentation (weeks)a | 14.3 (9.2) |
Gestational age at birth (weeks)a | 37.5 (2.8) |
Birth weight (grams)a | 2,975.0 (825.0) |
Sex |
|
Male | 82 (41) |
Female | 118 (59) |
Affected side |
|
Right | 140 (70) |
Left | 54 (27) |
Bilateral | 6 (3) |
Delivery type |
|
Normal vaginal delivery | 152 (76) |
Instrumental delivery | 40 (20) |
Cesarean section | 8 (4) |
Place of delivery |
|
Home delivery by traditional birth attendant | 30 (15) |
Hospital delivery by midwife | 120 (60) |
Hospital delivery by doctor | 50 (25) |
Prolonged labor | 136 (68) |
Presentation |
|
Cephalic | 144 (72) |
Breech | 40 (20) |
Transverse | 16 (8) |
Shoulder dystocia | 136 (68) |
Maternal diabetes | 40 (20) |
Maternal age (years)a | 27.5 (6.8) |
Parity of mother |
|
Primipara | 156 (78) |
Multipara | 156 (78) |
aMean (standard deviation), all others are n (%) | |
Mean escape latency of acquisition day | Groups | ||||
NC | SC | ColC | Pre-SwE Exp | Post-SwE Exp | |
Days |
|
|
|
|
|
1st | 26.2 (2.3) | 30.6 (2.4) | 60.0 (0.0)b | 43.2 (1.8)b | 43.8 (1.6)b |
2nd | 22.6 (1.0) | 25.4 (0.6) | 58.9 (0.5)b | 38.6 (2.0)b | 40.5 (1.2)b |
3rd | 14.5 (1.8) | 18.9 (0.4) | 56.5 (1.2)b | 34.2 (1.9)b | 33.8 (1.0)b |
4th | 13.1 (1.7) | 17.5 (0.8) | 53.9 (0.7)b | 35.0 (1.6)b | 34.9 (1.6)b |
5th | 13.0 (1.2) | 15.9 (0.7) | 51.7 (2.0)b | 25.9 (0.7)b | 27.7 (0.9)b |
6th | 12.2 (1.0) | 13.3 (0.4) | 49.5 (2.0)b | 16.8 (1.1)b | 16.8 (0.8)b |
Average of acquisition days | |||||
5th and 6th | 12.6 (0.2) | 14.6 (0.8) | 50.6 (0.7)b | 20.4 (2.1)a | 22.4 (3.2)a |
NC indicates normal control; SC, Sham control; ColC, colchicine control; SwE, swimming exercise exposure. aP <0.05; bP <0.01. | |||||
Categories | Number (%) |
Sex |
|
Male | 36 (60.0) |
Female | 24 (40.0) |
Age in yearsa | 8.8 (4.2) |
Education |
|
Pre-school | 20 (33.3) |
Elementary school | 24 (40.0) |
Junior high school | 16 (26.7) |
Cancer diagnoses |
|
Acute lymphoblastic leukemia | 33 (55) |
Retinoblastoma | 5 (8.3) |
Acute myeloid leukemia | 4 (6.7) |
Non-Hodgkins lymphoma | 4 (6.7) |
Osteosarcoma | 3 (5) |
Hepatoblastoma | 2 (3.3) |
Lymphoma | 2 (3.3) |
Neuroblastoma | 2 (3.3) |
Medulloblastoma | 1 (1.7) |
Neurofibroma | 1 (1.7) |
Ovarian tumour | 1 (1.7) |
Pancreatic cancer | 1 (1.7) |
Rhabdomyosarcoma | 1 (1.7) |
aMean (standard deviation) | |



Test results | Disease | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||
Yes | No | ||||||
Reid’s score ≥ 5 | Positive | 10 | 15 | 37.0 | 94.5 | 40.1 | 93.8 |
Negative | 17 | 258 |
|
|
|
| |
Swede score ≥ 5 | Positive | 20 | 150 | 74.1 | 45.0 | 11.8 | 94.6 |
Negative | 7 | 123 |
|
|
|
| |
Swede score ≥ 8 | Positive | 3 | 21 | 11.1 | 92.3 | 12.5 | 91.3 |
Negative | 24 | 252 |
|
|
|
| |
a High-grade indicates a score of ≥5 in both tests; PPV indicates positive predictive value; NPV, negative predictive value | |||||||
Test | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
Reid’s score ≥ 5 | 37.0 | 94.5 | 40.0 | 93.8 |
Swede score ≥ 5 | 74.1 | 45 | 11.8 | 94.6 |
Swede score ≥ 8 | 11.1 | 92.3 | 12.5 | 91.3 |
Test | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
Reid’s score ≥ 5 | 37.0 | 94.5 | 40.0 | 93.8 |
Swede score ≥ 5 | 74.1 | 45 | 11.8 | 94.6 |
Swede score ≥ 8 | 11.1 | 92.3 | 12.5 | 91.3 |
Narakas classification | Total 200 (100%) | Grade 1 72 (36%) | Grade 2 64 (32%) | Grade 3 50 (25%) | Grade 4 14 (7%) |
Complete recoverya | 107 (54) | 60 (83) | 40 (63) | 7 (14) | - |
Near complete functional recovery but partial deformitya | 22 (11) | 5 (7) | 10 (16) | 6 (12) | 1 (7) |
Partial recovery with gross functional defect and deformity | 31 (16) | 7 (10) | 13 (20) | 10 (20) | 1 (7) |
No significant improvement | 40 (20) | - | 1 (1.5) | 27 (54) | 12 (86) |
aSatisfactory recovery bGrade 1, C5, 6, 7 improvement; Grade 2, C5, 6, 7 improvement; Grade 3, panpalsy C5, 6, 7, 8, 9, Grade 4, panpalsy with Hornon’s syndrome. | |||||
Narakas classification | Total 200 (100%) | Grade-1 72 (36%) | Grade-2 64 (32%) | Grade-3 50 (25%) | Grade-4 14 (7%) |
Complete recoverya | 107 (54) | 60 (83) | 40 (63) | 7 (14) | - |
Near complete functional recovery but partial deformitya | 22 (11) | 5 (7) | 10 (16) | 6 (12) | 1 (7) |
Partial recovery with gross functional defect and deformity | 31 (16) | 7 (10) | 13 (20) | 10 (20) | 1 (7) |
No significant improvement | 40 (20) | - | 1 (1.5) | 27 (54) | 12 (86) |
aSatisfactory recovery bGrade 1, C5, 6, 7 improvement; Grade 2, C5, 6, 7 improvement; Grade 3, panpalsy C5, 6, 7,8,9, Grade 4, panpalsy with Hornon’s syndrome. | |||||
Variables in probe trial day | Groups | ||||
NC | SC | ColC | Pre-SwE Exp | Post-SwE Exp | |
Target crossings | 8.0 (0.3) | 7.3 (0.3) | 1.7 (0.2)a | 6.0 (0.3)a | 5.8 (0.4)a |
Time spent in target | 18.0 (0.4) | 16.2 (0.7) | 5.8 (0.8)a | 15.3 (0.7)a | 15.2 (0.9)a |
NC indicates normal control; SC, Sham control; ColC, colchicine control; SwE, swimming exercise exposure. aP <0.01. | |||||
Pain level | Number (%) | P | ||
Pre | Post 1 | Post 2 | ||
Mean (SD)a pain score | 4.7 (1.9) | 2.7 (1.6) | 0.8 (1.1) | <0.001 |
Pain categories | ||||
No pain (0) | - | 1 (1.7) | 31 (51.7) | <0.001 |
Mild pain (1-3) | 15 (25.0) | 43 (70.0) | 27 (45.0) | |
Moderete pain (4-6) | 37 (61.7) | 15 (25.0) | 2 (3.3) | |
Severe pain (7-10) | 8 (13.3) | 2 (3.3) | - | |
aPain scores according to the visual analogue scale ranging from 0 to 10; SD indicates standard deviation | ||||
Surgeries | Number (%) | Satisfactory outcomes n (%) |
Primary surgery (n=24) |
|
|
Upper plexus | 6 (25) | 5 (83) |
Pan-palsy | 18 (75) | 6 (33) |
All | 24 (100) | 11 (46) |
Secondary Surgery (n=26) |
|
|
Shoulder deformity | 15 (58) | 13 (87) |
Wrist and forearm deformity | 11 (42) | 6 (54) |
All | 26 (100) | 19 (73) |
Primary and secondary surgery | 50 (100) | 30 (60) |
Mallet score 14 to 25 or Raimondi score 2-3 or Medical Research grading >3 to 5. | ||
Narakas classification | Total 200 (100%) | Grade-1 72 (36%) | Grade-2 64 (32%) | Grade-3 50 (25%) | Grade-4 14 (7%) |
Complete recoverya | 107 (54) | 60 (83) | 40 (63) | 7 (14) | - |
Near complete functional recovery but partial deformitya | 22 (11) | 5 (7) | 10 (16) | 6 (12) | 1 (7) |
Partial recovery with gross functional defect and deformity | 31 (16) | 7 (10) | 13 (20) | 10 (20) | 1 (7) |
No significant improvement | 40 (20) | - | 1 (1.5) | 27 (54) | 12 (86) |
aSatisfactory recovery bGrade 1, C5, 6, 7 improvement; Grade 2, C5, 6, 7 improvement; Grade 3, panpalsy C5, 6, 7,8,9, Grade 4, panpalsy with Hornon’s syndrome. | |||||
Trials | Groups | ||||
NC | SC | ColC | Pre-SwE Exp | Post-SwE Exp | |
1 | 20.8 (0.6) | 22.1 (1.8) | 41.1 (1.3)b | 31.9 (1.9)b | 32.9 (1.8)a, b |
2 | 10.9 (0.6) | 14.9 (1.7) | 37.4 (1.1)b | 24.9 (2.0)b | 26.8 (2.5)b |
3 | 8.4 (0.5) | 9.9 (2.0) | 32.8 (1.2)b | 22.0 (1.4)b | 21.0 (1.4)b |
4 | 7.8 (0.5) | 10.4 (1.3) | 27.6(1.1)b | 12.8 (1.2)b | 13.0 (1.4)b |
Savings (%)c | 47.7 (3.0) | 33.0 (3.0) | 10.0 (0.9)b | 23.6 (2.7)b | 18.9 (5.3)b |
NC indicates normal control; SC, Sham control; ColC, colchicine control; SwE, swimming exercise exposure. aP <0.05; bP <0.01. cThe difference in latency scores between trials 1 and 2, expressed as the percentage of savings increased from trial 1 to trial 2 | |||||


Lesion-size | Histopathology report | Total | |||||
CIN1 | CIN2 | CIN3 | ICC | CC | SM | ||
0–5 mm | 73 | 0 | 0 | 0 | 5 | 5 | 83 |
6–15 mm | 119 | 18 | 1 | 4 | 0 | 0 | 142 |
>15 mm | 1 | 8 | 31 | 23 | 12 | 0 | 75 |
Total | 193 | 26 | 32 | 27 | 17 | 5 | 300 |
CIN indicates cervical intraepithelial neoplasia; ICC, invasive cervical cancer; CC, chronic cervicitis; SM, squamous metaplasia | |||||||
| Histopathology report | Total | ||||||
CIN1 | CIN2 | CIN3 | ICC | CC | SM | |||
Lesion -Size | 0-5 mm | 73 | 0 | 0 | 0 | 5 | 5 | 83 |
6-15 mm | 119 | 18 | 1 | 4 | 0 | 0 | 142 | |
>15 mm | 1 | 8 | 31 | 23 | 12 | 0 | 75 | |
Total | 193 | 26 | 32 | 27 | 17 | 5 | 300 | |
CIN indicates Cervical intraepithelial neoplasia; ICC, Invasive cervical cancer; CC, Chronic cervicitis; SM, Squamous metaplasia | ||||||||
Group | Didactic posttest marks (%) | Flipped posttest marks (%) | Difference in marks (mean improvement) | P |
<50% | 63.2 (9.4) | 82.2 (10.8) | 19.0 | <0.001 |
≥50% | 72.4 (14.9) | 84.2 ( 10.3) | 11.8 | <0.001 |
Data presented as mean (standard deviation) | ||||





Discussion
This study reveals that, within 3 weeks of onset in an acute rehabilitation setting, haemorrhagic strokes are associated with significantly greater disability than ischaemic strokes. The mean BI score for ischaemic stroke patients (62.0) corresponds to moderate to severe dependency on the BI scale, while the haemorrhagic group’s score (24.6) reflects total to severe dependency. These findings challenge the common assumption that ischaemic strokes result in greater disability during the acute phase, likely due to the more severe neurological impact of intracerebral haemorrhage.
The higher disability observed in haemorrhagic strokes aligns with previous studies. For example, Nakao et al. [8] reported lower BI scores in haemorrhagic stroke (21.5 ± 28.8) compared to ischaemic stroke (51.3 ± 36.6; P = 0.001). The greater disability in haemorrhagic stroke patients may be attributed to larger hematoma volumes and cerebral oedema, which cause more extensive neurological damage [9].
The predominance of total dependency among haemorrhagic stroke patients (52.8%) versus severe dependency in ischaemic stroke patients (64.1%) underscores the need for intensive early rehabilitation interventions tailored to haemorrhagic stroke survivors. Bathing, bladder control, and stair climbing emerged as the most consistently impaired domains, highlighting critical targets for rehabilitation aimed at improving mobility and personal care.
Demographically, the male predominance (2:1) and mean age (58.0 years) are consistent with regional studies [9]. The higher prevalence of right-sided hemiplegia (59%) contrasts with some studies reporting left-sided predominance, which may reflect sample characteristics or local epidemiological variations [10]. Hypertension as the leading risk factor (62.7%) aligns with global and local data, emphasizing its role in stroke prevention [11].
The study’s findings also highlight the predictive value of early BI scores. Granger et al. [12] identified a BI score of 60 as a threshold indicating transition from dependence to assisted independence, suggesting that ischaemic stroke patients, with a mean BI of 62, may have better potential for recovery compared to haemorrhagic stroke patients. The greater impairment in bladder control observed among haemorrhagic stroke patients highlights the need for targeted interventions, such as timed voiding schedules or pharmacological management. The consistently lower Barthel Index scores observed among older adults, particularly those with haemorrhagic stroke, likely reflect age-related frailty, higher comorbidity burden, and reduced physiological reserve, underscoring the need for age- and stroke-specific rehabilitation strategies.
The sample size was determined by consecutive enrolment during the study period, and no formal power calculation was conducted, which may limit the generalizability of findings. This study did not adjust for potential confounders such as age or comorbidities, which may influence disability outcomes.
Conclusion
Haemorrhagic stroke patients experience more severe disability than ischaemic stroke patients within 3 weeks of onset, as evidenced by lower BI scores and higher rates of total dependency. Bathing, bladder control, and stair climbing are critical areas for intervention. These findings advocate for early, intensive rehabilitation tailored to stroke type to optimize functional outcomes. Larger, multi-centre studies are needed to validate and expand these insights.



